Sterilisers
In general practices and other office- and community-based practices, sterilisation of instruments should be achieved by steam sterilisation under pressure (autoclaves). Other sterilisation systems that use dry heat, ionising radiation, ethylene-oxide, peracetic acid or hydrogen peroxide plasma sterilisation are not generally applicable due to cost, size or complexity.
Routine cycle monitoring and validation are required for all sterilisers to ensure they are performing correctly. The practice must also ensure that the steriliser is operated and maintained according to the practice’s documented procedures, based on the manufacturer’s instructions.
Under Therapeutic Goods Administration regulations, all new devices sold in Australia must perform the essential functions of their intended purpose. Practices must only use a steriliser model with an Australian Register of Therapeutic Goods certificate. Older sterilisers without certification should be replaced.
Steam sterilisers are classified according to the type of cycles they perform (class N, S or B). Dry-heat sterilisers must no longer be used.
Benchtop steam sterilisers (autoclaves)
Steam under pressure kills/inactivates organisms on the surfaces of the reusable medical equipment by using the latent heat of condensation to coagulate protein. The use of steam under pressure is the most reliable method for sterilising cleaned reusable medical devices.
Steam sterilisers are recommended as the method of choice for sterilisation of items in general practices and other office- and community-based practices.
Benchtop and portable sterilisers sold in Australia must be listed on the Australian Register of Therapeutic Goods. Sterilisers must also comply with state or territory regulations.
‘Small steam sterilisers’ are benchtop (portable) sterilisers that are unable to accommodate a sterilisation module and have a chamber volume of less than 60 litres.
Steriliser classification
Sterilisers are classified according to the types of cycles they can run (Table 10.6. Steriliser types). Some sterilisers can run more than one type of cycle.
Class S cycles
Class S cycles are defined as those provided by sterilisers that have an active drying cycle and are capable of sterilising unwrapped solid goods and at least one of the following:
- porous products (limited/no relevance to office-based practice)
- small porous items (such as gauze swabs; limited relevance to office-based practice)
- narrow-lumen (hollow A) items: long, thin hollow items – these are not sterilisable by most sterilisers that provide Class S cycles and are generally reprocessed using a Class B cycle
- simple hollow items (hollow B): shorter hollow items such as punch biopsy tips
- single wrapped products
- multiple wrapped products.
The use of Class S cycles is adequate for most general practices and other office- and community-based practices. Various steriliser designs can provide Class S cycles (Table 10.6. Steriliser types).
Gravity (downward displacement) sterilisers are the easiest to monitor and the most cost-efficient.
Table 10.6. Steriliser types
|
Type
|
Class
|
Description
|
Example of temperature -pressure curve
|
|
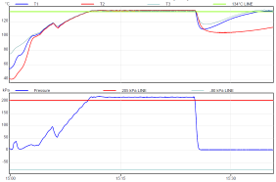
Gravity (downward displacement) and active drying
|
N
|
Traditional type
Relatively slow drying phase
Air removal by continual release of air/steam mixture via chamber drain line (eg back to internal water reservoir)
|
|
|
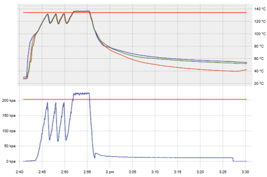
Purge under pressure (assisted air removal)
|
S
|
Air removal by alternating inflow and outflow of steam
|
|
|
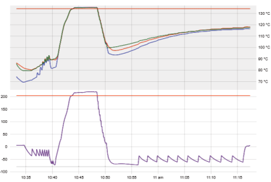
Single vacuum pulse (pre-vacuum, preliminary vacuum, vacuum assisted)
|
S
|
Air removal assisted by pump before sterilisation stage
Drying time reduced by active (‘post-vacuum’) removal of steam by pump
|
|
|
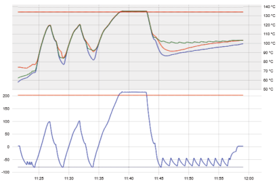
Multiple vacuum pulse (fractionated)
|
B
|
Complete air removal via vacuum pump in multiple vacuum pulses before the sterilisation phase, to ensure complete air removal and steam penetration into complex hollow and porous items. Pulsed vacuum is also used in the drying phase to reduce the time and temperature during drying to ensure the porous / wrapping/ packaging is dry at the end of the cycle
|
|
Class B cycles
Class B classes are cycles capable of sterilising:
Class B cycles are suitable for sterilising very long narrow hollow devices, which are rarely used in general practices and most other types of office-based practices. Class B sterilisers are commonly used in dentistry due to the frequent use of long, hollow reusable medical devices and the need for rapid processing of instruments.
In this type of steriliser cycle, air is removed by one or more vacuum stages to vent the air and allow in pressurised steam under pressure (fractionated vacuum system). Chamber pressures vary between types of systems: above and below atmospheric pressure (trans-atmospheric), above atmospheric pressure (supra-atmospheric) or below atmospheric (sub-atmospheric).
Sterilisers with Class B cycles (fractionated vacuum) are the most expensive to buy and operate and may require an onsite supply of deionised water.
Table 10.7. Definitions of hollow medical devices
|
Type
|
Definition
|
Examples
|
|
Not hollow
|
Ratio of the length of the cavity to the diameter is <1
|
Kidney dish
Bowl
|
|
Simple hollow
|
Single-ended open-space items where the ratio of length to diameter of the cavity is ≥1 and ≤5 and where the diameter is ≥5 mm
or
Double-ended open-space items where the ratio of the length to diameter of the cavity is ≥2 and ≤10 and where the diameter is ≥5 mm.
|
Punch biopsy tip
|
|
Narrow lumen
|
Hollow device beyond the range for a simple hollow item, and neither solid nor porous
|
Hormone implant insertion device
|