Case
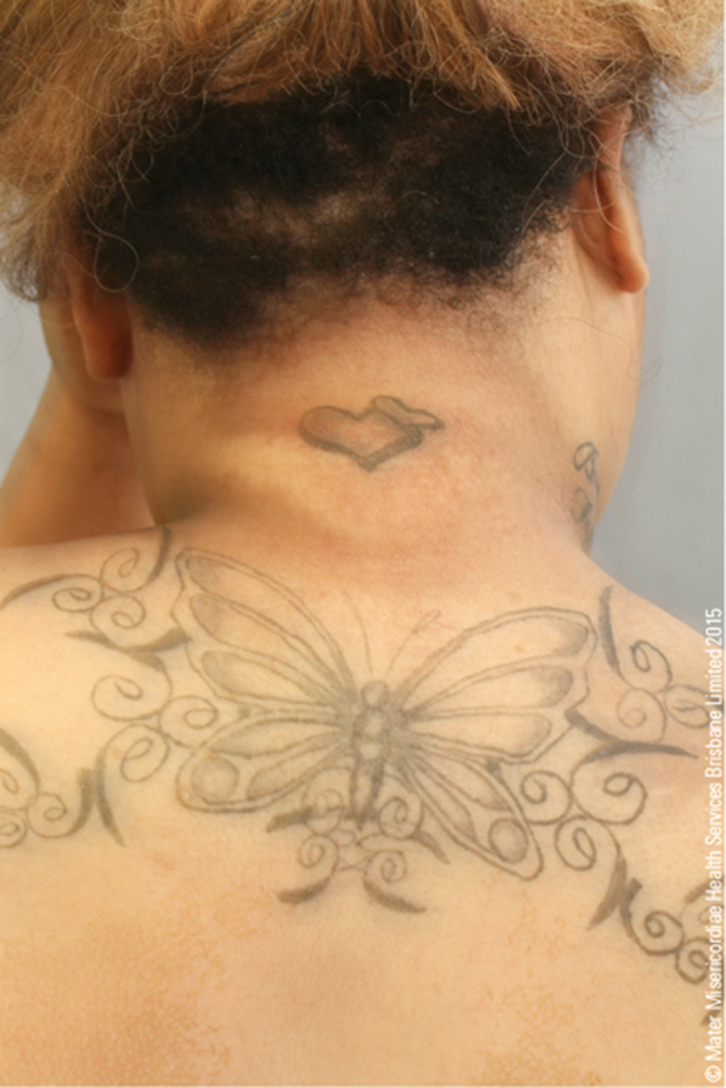
A Congolese refugee, 24 years of age, was referred to the emergency department with a random venous glucose of 29 mmol/L. She was noted to have pigmented striae, a buffalo hump, acanthosis nigricans and moon face (Figures 1, 2).
 |
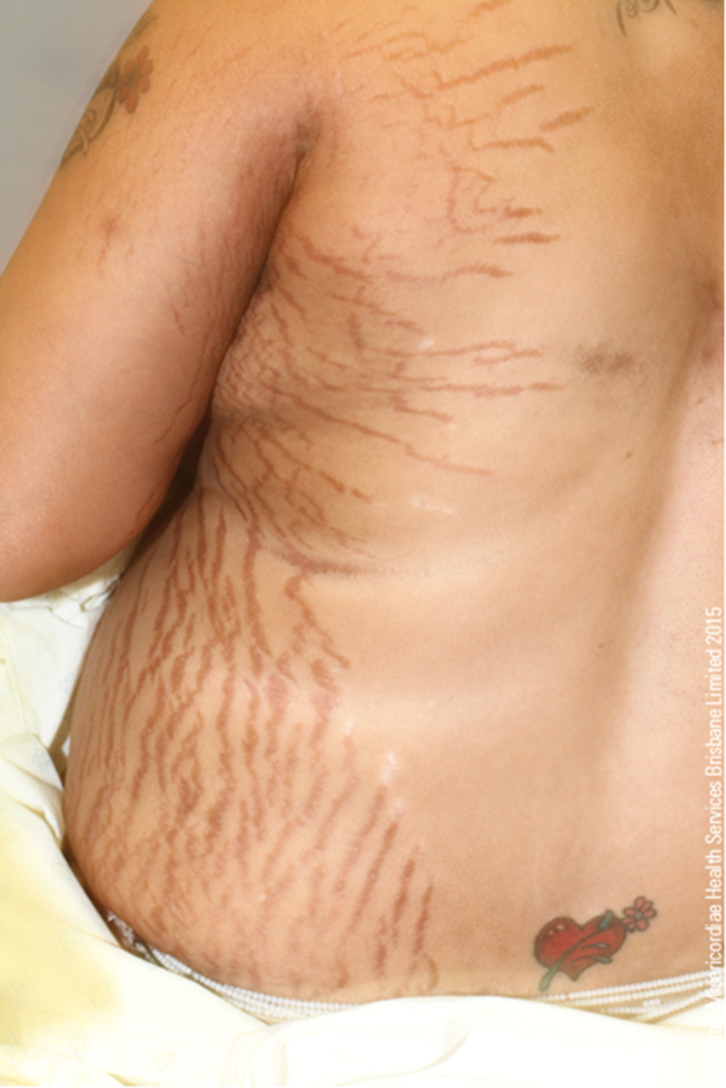 |
Figure 1. Posterior neck showing buffalo hump
and acanthosis nigricans |
Figure 2. Left flank with cutaneous striae |
Question 1
What is the likely cause of the patient’s appearance and hyperglycaemia?
Question 2
How would you confirm the diagnosis?
Answer 1
The likely cause of the patient’s appearance and blood glucose level is Cushing’s syndrome.
Answer 2
A 24-hour urine free cortisol test or overnight dexamethasone suppression test is needed to confirm a diagnosis of Cushing’s syndrome.
Case continued
The patient’s serum cortisol and plasma adrenocorticotropic hormone (ACTH) levels, and urinary cortisol-to-creatinine ratio were below the lower detection limit of the respective assays.
Question 3
What is the likely aetiology of her condition?
Answer 3
The patient’s test results are consistent with hypothalamic-pituitary-adrenal (HPA) axis suppression, which suggests that the Cushingoid appearance and increased blood glucose levels were due to the use of exogenous glucocorticoids.
Case continued
The patient denied using any medications other than a topical cream she had obtained over the counter from a local African shop for the purpose of skin lightening.
Question 4
How would you investigate the source of exogenous glucocorticoids?
Answer 4
Mass spectrometry of the patient’s serum and the cream detected clobetasol, a potent corticosteroid. Prednisolone and dexamethasone were not detected.
Discussion
At least 45 cases of iatrogenic Cushing’s syndrome resulting from the use of a very potent topical corticosteroid have been reported.1–3 Of those affected, 23 were children, predominantly infants treated for diaper dermatitis (83%). Two of these infants died from severe disseminated cytomegalovirus infection. The majority of cases (73%) in adults were patients receiving topical corticosteroids for psoriasis. Clobetasol was the topical corticosteroid responsible in 78% of these patients, and betamethasone in 18% of cases. The median duration of topical corticosteroid usage prior to the diagnosis of Cushing’s syndrome was 2.75 months in children and 18 months in adults. At least eight cases in which Cushing’s syndrome developed as a result of topical corticosteroid use for skin lightening have been published.3–9 Notably, the majority of these cases occurred in people who had migrated from Africa to Australia, Canada, the United States or Europe. In addition, Raynaud et al reported a significantly higher prevalence of diabetes mellitus and hypertension in women using depigmenting agents in Senegal.10
The use of topical corticosteroids for skin bleaching has been reported in 25–67% of adult women in western African capital cities.11 Sixty-nine per cent of women attending a standard maternal centre in Dakar used skin lighteners in the third trimester of pregnancy. Lighter skin tone may be associated with improved self-esteem, perception of youth and beauty, and marriage and employment opportunities.
Skin lightening products may contain potent glucocorticoids, including clobetasol propionate, betamethasone propionate and fluticasone propionate. The severity of complications with topical corticosteroid use depends on the extent, quantity and duration of application, potency of medication, permeability of the skin, and age of the person. Cushing’s syndrome in children manifests predominantly as obesity and growth arrest. In adults, the typical Cushingoid appearance, together with diabetes mellitus, hypertension, osteoporosis, oedema and irregular menses, may occur.
In addition, topical corticosteroid use may be associated with HPA axis insufficiency, with low or suppressed levels of serum cortisol and plasma ACTH. The mean time to recovery of HPA axis function after cessation of topical corticosteroids was 3.49 ± 2.92 months (range 1–12 months) in children, and 3.84 ± 2.51 months (0.5–10 months) in adults.1 During this recovery time, patients require physiological replacement with oral glucocorticoids, appropriate advice regarding increasing the dose with intercurrent illness, and parenteral replacement in the event of vomiting. The use of skin lightening products containing potent topical corticosteroids in pregnancy was associated with smaller placentas and a higher rate of low-birth-weight infants.11
In Australia, prescription of highly potent topical corticosteroids is restricted to specialist prescribers. The patient in this case had obtained clobetasol at a local African shop, the proprietor having imported supplies from overseas. Highly potent topical corticosteroids may also be obtained via the internet.
In conclusion, a case of Cushing’s syndrome and HPA axis suppression due to the use of potent topical corticosteroids for the purpose of skin lightening is presented. General practitioners should be aware of this association given the high reported prevalence of use of skin lightening creams by African women.
Authors
Adam Morton FRACP, Endocrinologist, Mater Health Services, Brisbane, QLD. adam.morton@mater.org.au
Anish Menon FRACP, Endocrinologist, Princess Alexandra Hospital, Brisbane, QLD
Trisha O’Moore-Sullivan FRACP, Director, QLD Diabetes and Endocrine Centre, Mater Health Services, Brisbane, QLD
Competing interests: None.
Provenance and peer review: Not commissioned, externally peer reviewed.