It is well recognised that dialysis is a costly treatment.1 On fiscal and quality-of-life bases, it is preferable for dialysis to be delayed or, if possible, averted altogether. Integrated care is an essential requirement for optimising the management of chronic kidney disease (CKD). Identifying at what point in the disease process various interventions will have the greatest management impact is essential.
Research to date suggests that the role of the primary care provider will be paramount in renal medicine, and opportunistic screening of high-risk groups appears to be a cost-effective strategy.2 The introduction of estimated glomerular filtration rates (eGFR) in 2005 has been instrumental in facilitating the detection of CKD.3 Enhanced detection has come with an increase in referrals, a feature not restricted to Australia, with similar findings published internationally.4–6
Established national referral guidelines aim to assist primary care providers to quickly identify patients who are in need of specialist management. As in the US, UK and Canada,7–9 the degree of chronic renal impairment that would trigger specialist nephrology involvement in Australia is suggested as Stage 4 CKD (eGFR <30 ml/min/1.73 m2).10 In an ideal system, patients with less advanced CKD would not require consultation with a nephrologist. However, this is often less clearly demarcated for the primary care physician who may be faced with a patient who does not meet exact referral guideline criteria, but whose management is not straightforward.
The general practitioner (GP) may be seeking clarification around a single issue, but in the present system, can only access this advice by initiating a formal referral or attempting to directly contact the physician for advice. This system is problematic from all perspectives. Contacting the physician directly is often a frustrating, time-consuming process. Even if successful, the GP may find that the physician is in the midst of another task, with attention and focus divided. Booking the patient into the clinic extends the waiting period and makes triaging more urgent cases difficult. The GP and patient are frustrated by long waiting periods. The patient often requires time off work, with subsequent decreased productivity; greater disruption ensues if carer and transport requirements arise. With this in mind, we sought to define and describe referrals made to the Nephrology Department at St George Hospital in Sydney over a three-year period. This was in order to assist in planning a more efficient system, and providing a dynamic interface between the primary care provider and nephrologist.
Methods
This retrospective study was conducted at St George Hospital, a tertiary referral hospital in south-east Sydney, New South Wales, Australia. Ethics approval was obtained from the Human Research Ethics Committee of the South Eastern Sydney Local Health District (LNR/11/STG/187).
Two hundred randomly selected nephrology outpatient referrals made to the renal service at St George Hospital between January 2008 and December 2011 were reviewed. This represented 20% of the total cohort seen in this period. Private practice referrals were not assessed in this study. Of the total cohort, referrals originating from another specialist or generated as follow-up from an earlier inpatient admission were excluded from the study. The sampling process involved taking every fifth file after the files were arranged in order of presentation.
Referrals were retrospectively analysed and classified into seven groups according to the primary practitioner’s stated motivation for referral:
- Stage 4 CKD
- Declining renal function
- Control of hypertension
- Isolated proteinuria
- Isolated haematuria
- Recurrent urinary tract infections
- ‘Other’ (which incorporated recurrent stone disease, renal cysts, hypotension, abnormal renal imaging, abnormal biochemistry, polyuria and hypoalbuminaemia)
The referrals were then assessed on the basis of the accompanying pathology results for the patient’s stage of CKD, declining renal function, presence of hypertension and evidence of renal disease activity (using surrogate markers). Medications, blood pressure and urinalysis at the time of referral (or first consultation if unavailable at time of referral) were documented.
The referrals were then assessed against Kidney Health Australia’s referral guidelines in Chronic kidney disease management in general practice 2007 operating at the time of the study.11 These guidelines represent locally based expert assessment of the available literature. Recommendations are based on the assessment of available evidence by the Kidney Check Australia Task Force (KCAT) scientific advisory committee, which comprises nephrologists, GPs, consumers and nurse specialists. Preliminary investigations following the initial clinic review and the number of follow-up clinic visits required prior to discharging the patient back to referring practitioner were also recorded.
Statistics
Data were analysed using IBM’s SPSS statistics (version 20; SPSS Inc, Chicago, IL, US). Descriptive statistics were performed and measures of variability are expressed as mean, standard deviation or percentage.
Results
Within the three-year study period, 22 of the 200 randomly selected referrals to the public clinic were excluded. Fourteen referrals had originated from another specialist and eight were generated from follow-up of a patient who had received a nephrology consultation while an inpatient. Patient characteristics of the remaining 178 are described in Table 1.
Table 1. Patient characteristics at referral (n = 178)
|
| | Mean ± SD or % (n) |
|---|
| Age (years) |
59 ± 19 |
| Gender (female) |
56 (99) |
| Weight (kg) |
77 ± 18 |
| Clinic SBP (mmHg) |
134 ± 31 |
| Clinic DBP (mmHg) |
79 ± 17 |
| Creatinine (µmol/L) |
116 ± 66 |
| Creatinine (mg/dl) |
1.31 ± 0.75 |
| CKD stages | % (n) |
|---|
| Stage 1 (eGFR >90 ml/min/1.73 m2 ) |
20 (35) |
| Stage 2 (eGFR 60–89 ml/min/1.73 m2) |
23 (41) |
| Stage 3a (eGFR 46–59 ml/min/1.73 m2) |
19 (34) |
| Stage 3b (eGFR 30–45 ml/min/1.73 m2) |
21 (38) |
| Stage 4 (eGFR 15–29 ml/min/1.73 m2) |
11 (19) |
| Stage 5 (eGFR <15 ml/min/1.73 m2) |
1 (1) |
| No creatinine provided |
5 (10) |
|
CKD, chronic kidney disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; SBP, systolic blood pressure; SD, standard deviation
|
A stated decline in renal function accounted for 44% (n = 78) of referrals (Figure 1). Ten per cent of these patients had a glomerular filtration rate of greater than 60 ml/min. The duration and degree of decline was compared with KHA’s referral recommendations, existing at the time vis-a-vis greater than 15% decline in eGFR over at least a three-month period.11 Using this criterion, only 44% of these patients (n = 34) fulfilled the criteria for specialist review and just more than a third of those referred with a stated decline in renal function (n = 25) were referred back to the primary carer within six months of initial referral (Table 2).
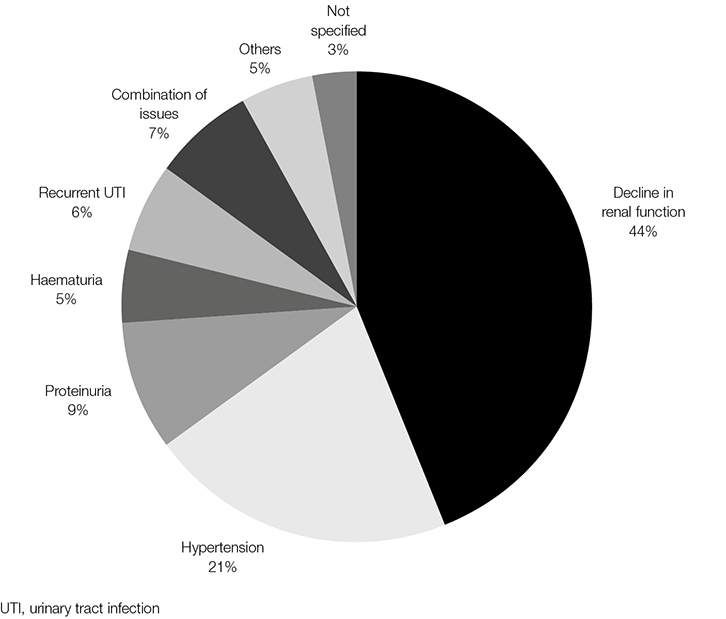 |
| Figure 1. Nominated basis for referral (n = 178) |
Table 2. Appropriateness of referrals according to Kidney Check Australia Taskforce (KCAT) guidelines for indications for referral to a nephrologist15
|
| Referral reason (KHA criteria, 2007) | | |
|---|
| |
Met referral criteria
Total n = 125
% (n) |
Did not meet referral criteria
Total n = 52
% (n) |
| Patients referred for decline in renal function eGFR <30 ml/min/1.73 m2 |
15 (19) |
|
| Unexplained decline in kidney function (>15% drop in eGFR over three months) |
27 (34) |
46 (24) |
| Significant proteinuria >1 g/24 hours* |
11 (14) |
6 (3) |
| Glomerular haematuria |
8 (9) |
|
| eGFR <60 ml/min/m2 and uncontrolled hypertension |
4 (5) |
|
| Diabetes and eGFR <60 ml/min/1.73 m2 |
20 (25) |
|
| Other reasons (recurrent UTI, renal calculi, renal cysts ) |
11 (14) |
|
| eGFR >60 ml/min/1.73 m2 and uncontrolled hypertension with more than three antihypertensives |
4 (5) |
48 (25) |
|
*Or protein-to-creatinine ration ≥100 mg/mmol or albumin-to-creatinine ratio ≥60 mg/mmol or 1+ dipstick protein
eGFR, estimated glomerular filtration rate; UTI, urinary tract infection
|
The next most frequent indication for referral (22%; n = 38) arose for the management of hypertension (Table 3). Of these patients, only 13% (n = 5) were on three or more antihypertensives, and of those with an available eGFR (n = 35), the majority (86%; n = 30) arose from CKD Stages 1 and 2 (eGFR ≥60 ml/min/1.73 m2). Each of the other referral categories comprised <10% of the sampled cohort, and the majority of these fulfilled national referral guidelines (Table 4).
Table 3. Characteristics of the primary referral groups
|
| | Decline in
renal function
(n = 78)
% (n) | Hypertension
(n = 38)
% (n) | Proteinuria
(n = 17)
% (n) | Haematuria
(n = 9)
% (n) | Recurrent UTI
(n = 10)
% (n) |
|---|
| CKD stages* |
|
|
|
|
|
|
Stage 1 (eGFR >90 ml/min/1.73 m2)
|
|
47 (18) |
29 (5) |
56 (5) |
40 (4) |
|
Stage 2 (eGFR 60–89 ml/min/1.73 m2)
|
10 (8) |
32 (12) |
24 (4) |
22 (2) |
10 (1) |
|
Stage 3a (eGFR 46–59 ml/min/1.73 m2)
|
23 (18) |
8 (3) |
29 (5) |
22 (2) |
10 (1) |
|
Stage 3b (eGFR 30–45 ml/min/1.73 m2)
|
42 (32) |
5 (2) |
6 (1) |
|
|
|
Stage 4 (eGFR 15–29 ml/min/1.73 m2)
|
23 (18) |
|
6 (1) |
|
|
|
Stage 5 (eGFR <15 ml/min/1.73 m2)
|
1 (1) |
|
|
|
|
| No creatinine provided |
1 (1) |
8 (3) |
6 (1) |
|
40 (4) |
| Dipstick protein positive |
46 (36) |
18 (7) |
82 (14) |
33 (3) |
30 (3) |
| Dipstick blood positive |
30 (23) |
26 (10) |
41 (7) |
100 (9) |
30 (3) |
Spot urinary protein and/or 24-hour
urine protein |
20 (16) |
|
53 (9) |
|
|
| <3 Antihypertensives or none |
76 (59) |
74 (28) |
71 (12) |
100 (9) |
90 (9) |
| ≥3 Antihypertensives |
24 (19) |
26 (10) |
29 (5) |
|
10 (1) |
|
*eGFR calculated using the Modification of Diet in Renal Disease (MDRD) formula
CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; UTI, urinary tract infections
|
Table 4. Patient discharge information within 12 months of initial clinic visit (n = 178)
|
| Original reason for referrals | Decline in renal function
(n = 78) | Hypertension
(n = 38) | Proteinuria
(n = 17) | Haematuria
(n = 9) | Recurrent UTI
(n = 10) | Combination
of issues
(n = 12) | Others
(n = 9) | Not specified
(n = 5) | Total
(%; n = 178) |
|---|
| One visit and discharged back to GP |
11 |
2 |
4 |
1 |
3 |
1 |
2 |
3 |
15% |
| Follow-up ≤6 months |
14 |
3 |
5 |
4 |
1 |
3 |
4 |
1 |
20% |
| Follow-up ≥7 months ≤12 months |
4 |
5 |
1 |
1 |
|
2 |
|
|
7% |
| On follow-up after 12 months |
40 |
20 |
4 |
2 |
2 |
3 |
1 |
|
40% |
| Lost to follow-up or relocated |
9 |
8 |
3 |
1 |
4 |
3 |
2 |
1 |
18% |
| Number of visits within 12 months (mean ± SD) |
3 ± 2 |
4 ± 2 |
2 ± 0.3 |
2 ± 1 |
2 ± 1 |
4 ± 2 |
2 ± 0.7 |
1 ± 0.4 |
|
Fifteen per cent (n = 27) of patients were discharged after their first attendance at the outpatient clinic and 35% (n = 62) of patients had care transferred back to their referring primary care practitioner within six months. Within the year, almost one in five had either moved out of the area or did not attend their scheduled follow-up appointments. Only 40% (n = 74) of the original referral cohort received ongoing nephrologist care at 12 months (Table 2).
Some of the common interventions that were carried out by the nephrologists were renal imaging, 24-hour ambulatory blood pressure monitoring (ABPM), dietitian referrals and 24-hour urine collections.
Discussion
As evidenced by the high rate of early discharge from the clinic, often after the first visit, primary care practitioners frequently require diagnostic or management advice that does not require lengthy investigations or review. Almost half of the patients were referred for falling eGFR, but just over 40% actually met the national referral guidelines for this parameter.
The national KHA guidelines represent the best available renal-based recommendations within an Australian framework. These, like all guidelines, do not claim perfection; rather, they provide a framework in which the GP and specialist can work. The grey zone of evidence highlights the need for a system that allows flexibility. Electronic or e-communication is one of many options available to bridge areas of uncertainty, and integrate care between the specialist and GP.
Results from this study are not provided to criticise the primary care provider referral pattern. Rather, this study provides data that highlights a GP’s need for an alternative referral process. Such a process would free traditional specialist interactions for those patients in need of a face-to-face review. A locally integrated, patient-centred, comprehensive strategy is required to achieve widespread and sustained improvements in the quality of care for people with chronic kidney disease in general practice.
There are many good examples of integration, and improved care would involve multiple components.12 Integrated information technology (IT) is one means by which all providers are able to access a patient’s health record, minimise duplication of tests and investigations, remain involved, and track progress and management decisions. However, other aspects would include aligning finances and responsibility, collaborative-care planning, effective clinical engagement, and governance.
All referrals reflect a need from the referring practitioner for clarification or guidance; as such, referrals are never regarded as inappropriate. Notwithstanding this, some referrals would be best served by a personal discussion with the GP rather than requiring a patient to attend an outpatient clinic for review. The early triage of patients by the nurse consultant, GP and specialist will ensure that, despite limited experience or expertise in a particular chronic disease such as CKD, the GP will have access to specialist knowledge early in the disease process.
Improved communication would be to the benefit of all stakeholders concerned. The GP could discuss their concerns and be reassured that the patient could continue to be managed in a primary care setting. The patient is saved time and finances incurred when travelling to see a specialist. The community benefits from fewer patients requiring time off work. Costs to the federal and state/territory governments are reduced as outpatient visits are minimised and specialist referrals reduced. Specialists are able to spend time in clinics with priority patients, and waiting times for such patients are minimised. Finally, important duties, such as teaching and training, which often suffer when clinics are overstretched, can be undertaken to a greater extent.
One proposed alternative delivery system currently being trialled within our unit is to provide primary care practitioners with electronic access to specialist opinions specific for their particular patient.13 The delivery system, through the South East Sydney Medicare Local, includes the involvement of a clinical nurse specialist to support general practices. It is built around a web-based IT system, but it also includes traditional methods of communication such as fax, telephone and email. This modification of service delivery dovetails well into chronic-care models.14,15 This service delivery provides an improved consultation delivery design, which could ultimately be integrated into existing clinical information systems and incorporate inter-professional input from allied health groups.
With large, centralised healthcare provision, a telephone call to discuss a patient and request advice may not always be the most efficient and optimal method. Time is wasted by the primary care provider contacting the specialist and, frequently, the specialist is focusing on an alternative problem at the time. In addition, either party may inadvertently overlook data relevant to the case. A far better system allows for management of investigations and exchange of data in a transparent, recordable and timely manner, attended to by a specialist dedicated to the case.
As evidenced by this retrospective, descriptive study, 15% of current referrals were referred back to the primary care provider after just one visit and 35% by six months. This suggests that if timely advice could have been provided, care may have been able to remain with the GP for at least half (50%) of all patients.
Countries with centralised healthcare systems based primarily in metropolitan areas typically have difficulty servicing patients in rural or remote areas.16 It is not just the delivery of medical care for acute events such as the management of myocardial infarctions, where distance from the specialist centre is a negative outcome factor.17 Patients with chronic diseases also fair better when access to healthcare is improved.18,19 Remote and rural GPs would gain much support from the proposed system. The system can relieve some of the mismatch that exists, at least in Australia, between specialist practice sites and populations. Such referral strategies will take on even greater importance as the anticipated CKD burden intensifies and have potential to improve service delivery to more remote regions.
This study is relatively small and draws from a tertiary referral hospital, factors that have a negative impact on generalisabilty. Although a small study, it is typical of many medical specialist fields traditionally serviced through a hospital outpatient clinic. Systems are required to modify the current cumbersome delivery of healthcare. This would relieve pressure on hospital clinics and improve the access of patients who require specialist review. At the same time, it would also provide timely support for the primary care physician. We are hopeful studies such as this compel the readership and administrative bodies to ongoing discussion in this area.
Authors
Cathie Lane BPharm, GradCert, ULTeaching, BMed, FRACP, PhD, Staff Specialist Nephrology, Department of Renal Medicine, St George Public Hospital, Kogarah, NSW; Conjoint Senior Lecturer, University of New South Wales, Sydney, NSW. cathie.lane@sesiahs.health.nsw.gov.au
Saiyini Pirabhahar BNurs, MHlthSci, GradDip Med Lab Sci, Renal Research Officer, Department of Renal Medicine, St George Public Hospital, Kogarah, NSW
Jennifer Robins MBBS, BSc (Hons), BMedSc, MPH, Gen Med/Nephrologist Visiting Medical Officer, Mona Vale Hospital, Mona Vale, NSW; and Visiting Medical Officer and Nephrologist, Mater Hospital and Mater Dialysis Unit, Sydney, NSW
Shelley Tranter RN, DN, MApSng, RenalCert MCN, Renal CNC, St George Public Hospital, Kogarah, NSW
Mark Brown MBBSm MDm FRACP, Professor of Medicine, University of New South Wales, Sydney, NSW
Ivor Katz MBBCh, BSc (Hons), PhD, Consultant Nephrologist, St George Public Hospital, Kogarah, NSW; Associate Professor, Faculty of Medicine, University of New South Wales, Sydney, NSW
Competing interests: None.
Provenance and peer review: Not commissioned, externally peer reviewed.