When considering burns dressings, it is useful to remember the theory of Jackson’s burn wound model (Figure 1). The inner zone of a burn is the zone of coagulation. This area is dead and nothing any clinician can do will bring it back to life. The outer zone of the burn is called the zone of hyperaemia. This is a reactive zone of inflammation in response to the injury, which can occur with non-burn injuries such as trauma, and will return to normal within hours of the injury. The middle zone of the burn is called the zone of stasis, which is the target of good burns care, such as effective first aid and dressings. Good first aid and wound management can significantly reduce the need for skin grafting,1 simply by giving this middle zone the chance to recover, rather than deepen and become part of the zone of coagulation. This model helps to explain the dynamic nature of burn injuries, and how an assessment of the burn at the time of injury can be different in terms of size and depth to an assessment of the same injury 48 hours later.
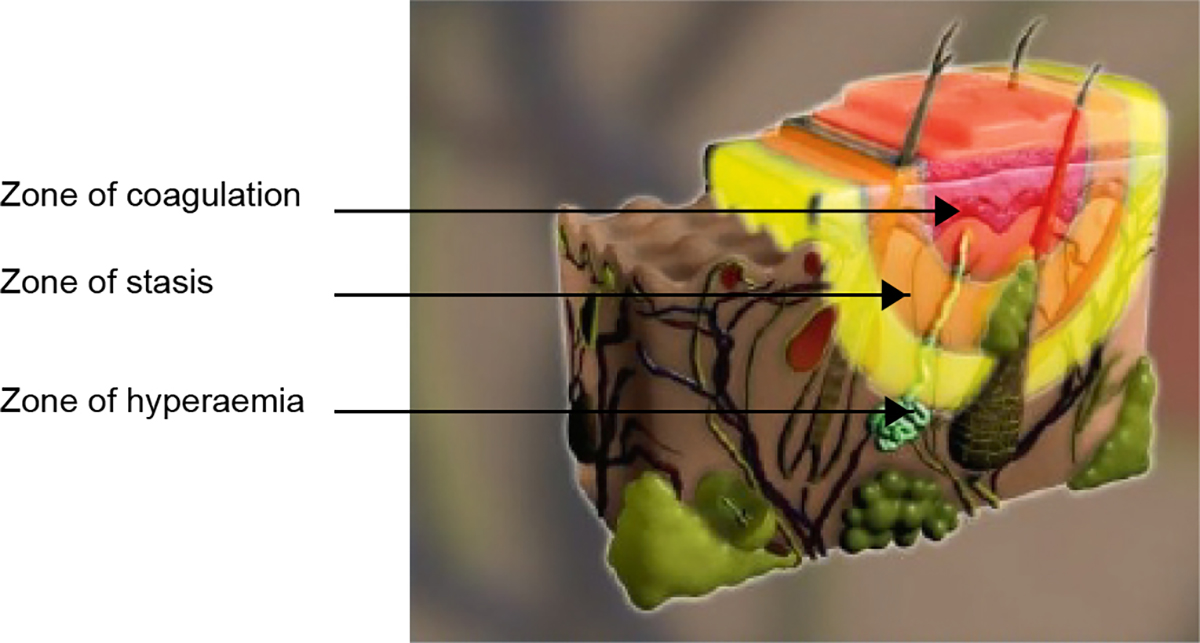
Figure 1. Jackson’s burn wound model
Reproduced with permission from Wound Healing Institute Australia. Jackson’s burn wound model. Perth: WHIA, 2017.
If the zone of coagulation is large, it is likely that the patient will require specialist treatment and surgery. However, good initial management, including dressings, can still prevent the burn from getting larger and deeper while the patient awaits review or transfer.
Aims of burns dressings
When considering the choice of dressing for a burn injury, it is important to think of the size and depth of the burn, and also the aim of the dressing to be applied.
A superficial epidermal burn (eg sunburn, minor scald, brief flash burn, where there is erythema of the skin but no break in the skin or blistering) does not require a dressing, and application of emollient or moisturiser to cool the intact but painful red skin is appropriate (Figure 2).
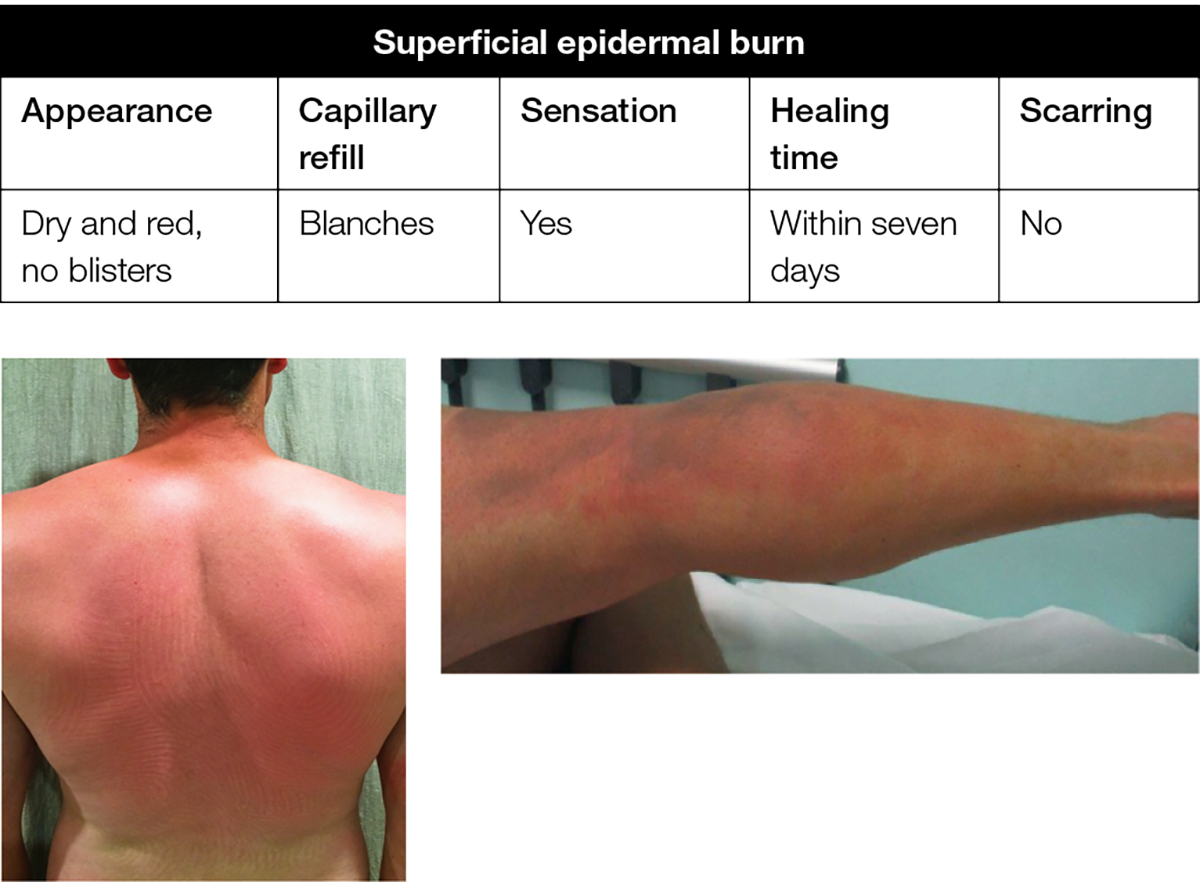
Figure 2. Superficial epidermal burn
A superficial dermal burn (eg hot water scald, where there is skin blistering over a wet, pink and painful dermis) requires a dressing to absorb fluid, avoid maceration and seal the wound from the outside environment to reduce pain and infection (Figure 3).
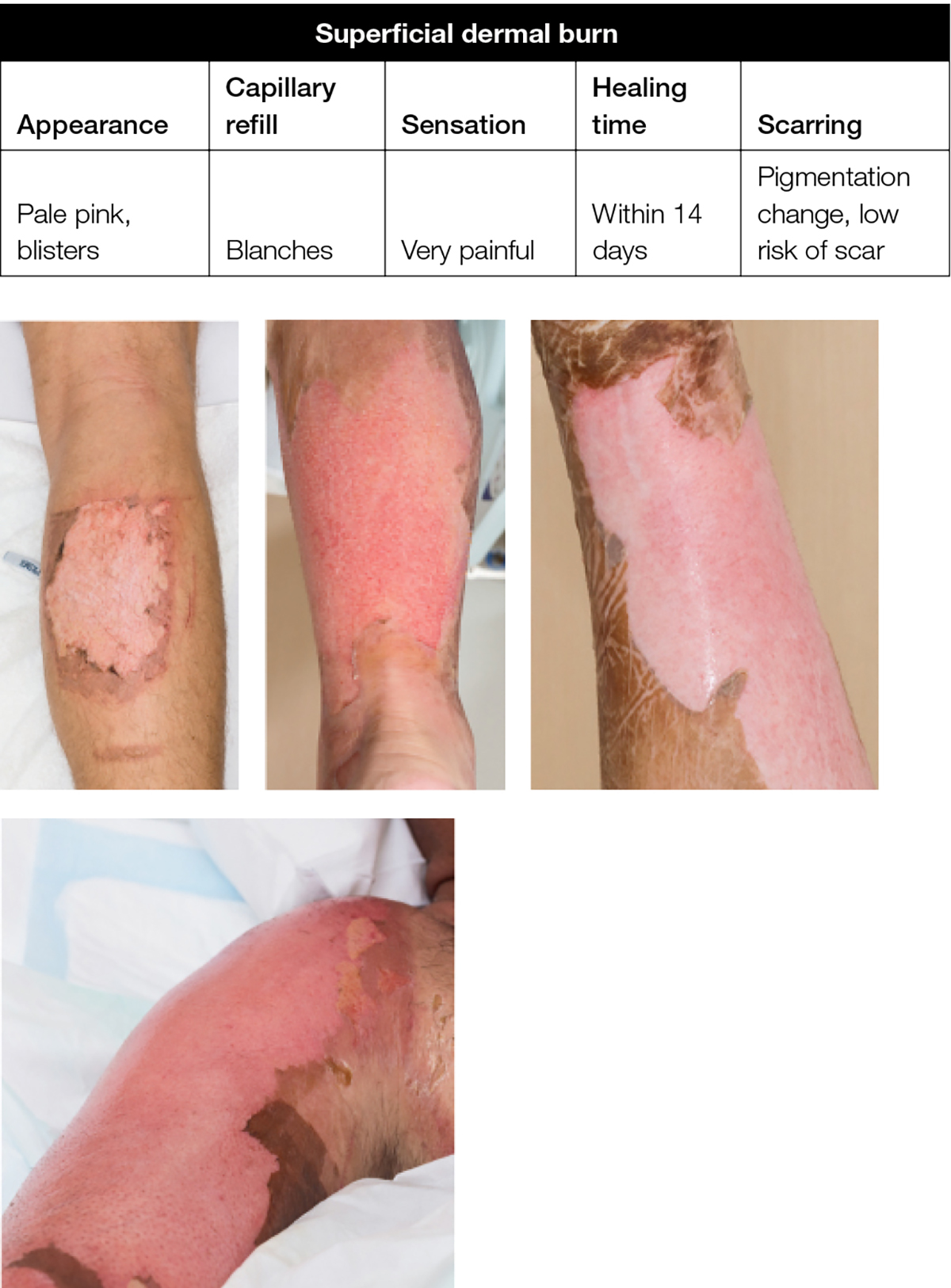
Figure 3. Superficial dermal burn
A deep dermal or full-thickness burn (eg prolonged flame, contact burn, where skin under the broken or destroyed blister is less painful and a fixed red or pale white colour due to damaged blood vessels, proteins and nerve endings) will require a dressing to debride and lift the dead skin if it is a small area, or to temporise for surgery if it is larger area (Figure 4).
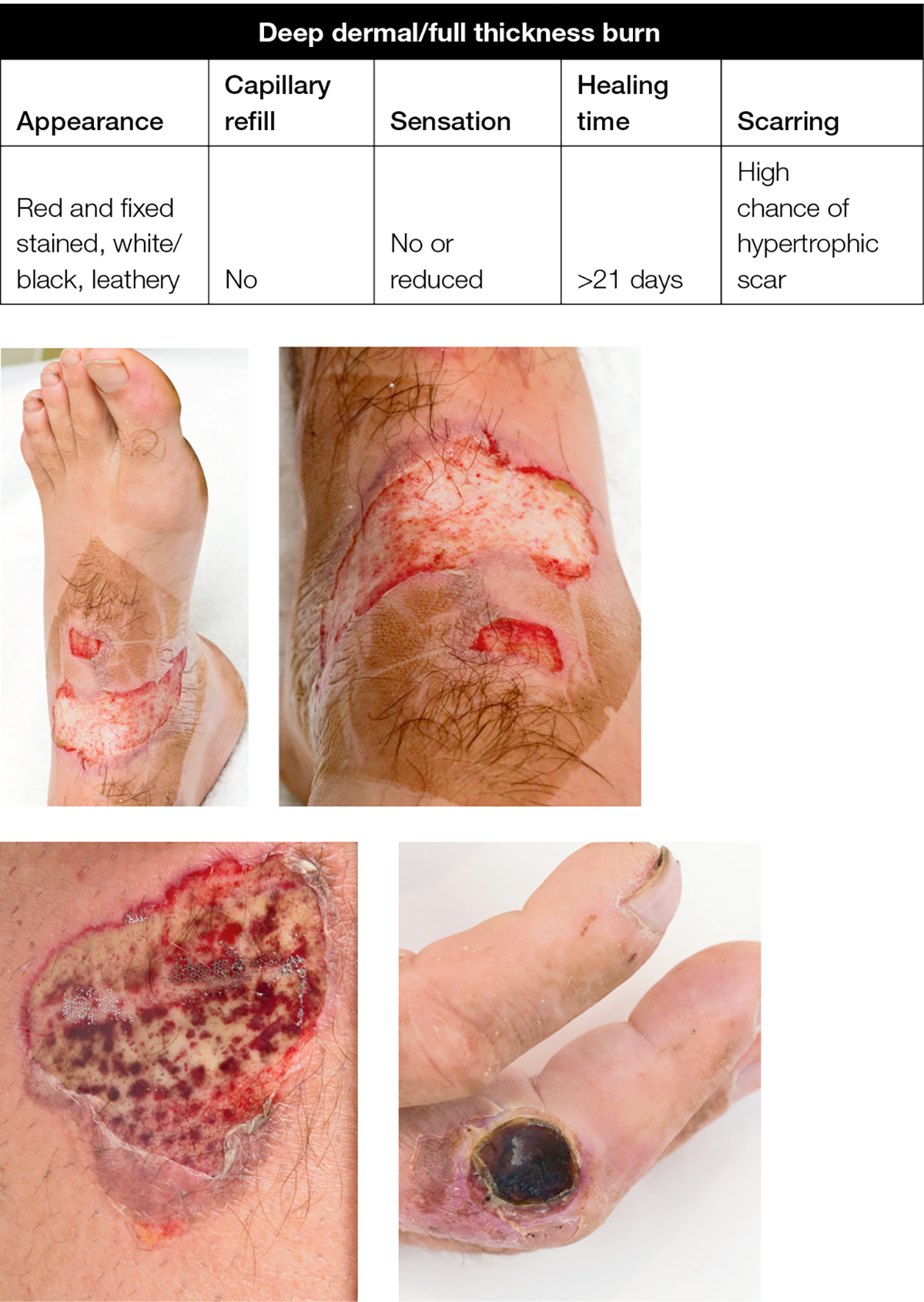
Figure 4. Deep dermal/full thickness burn
It follows, therefore, that different burns require different dressings. The overall aims of any burn wound dressing, irrespective of the size and depth of the burn, include:
- preventing infection
- promoting moist wound healing
- preventing conversion to a deeper burn
- reducing pain
- allowing for movement and function
- assisting in decreasing swelling.
Blister debridement
The management of blister debridement is still controversial; however, the authors’ burns service, in line with literature reviews of available evidence,2 supports the practice of blister debridement for the following reasons:
- The build-up of fluid under the intact blister (which becomes firm and jelly-like quite rapidly) can put pressure on the underlying dermis, which in turn can reduce perfusion and potentially deepen burns.
- Blister fluid contains thromboxane B2, a powerful vasoconstrictor that could reduce perfusion.2,3
- The blister skin is dead and should be removed as it is a potential focus for infection.
- The point of an antibacterial dressing is that it has contact with the viable skin – this is not possible if the blister is intact.
- Intact blisters are painful and reduce movement, which in turn increases swelling. Swelling of tissues increases the perfusion distance from capillaries to skin and this can reduce skin perfusion and deepen the burn.
If the treating clinician is not experienced or confident in blister debridement, then ‘snipping’ the top of the blister with sterile scissors is recommended to de-roof it, encourage egress of the blister fluid and relieve pain.
Initial burn dressings
Burn wounds are dynamic and change in appearance, particularly in the first 48 hours. Therefore, it is the practice of the authors’ burns service to review burns after 48 hours before decisions regarding definitive dressings or surgery are made. It follows, therefore, that the initial burn dressing should be one that can remain intact for 48 hours and prevent infection. The microbiology and infection risk in Australia is unique because of the very variable climate and prolonged transfer times in some rural areas to medical attention.4 The use of antimicrobial dressings in such an environment has been shown to improve outcomes by reducing infection.5,6 Nanocrystalline silver dressings (eg Acticoat), slowly release silver, which is toxic to microorganisms, to the burn wound bed. It is protocol in the authors’ burn service to dress all burns with this dressing for the first 48 hours. Practical tips for the use of nanocrystalline dressings include:
- moistening silver dressings with sterile water (not saline – the chloride ion could bind to the silver ion, reducing the amount of silver delivered to the wound)
- applying a secondary dressing on top
- wet gauze, followed by dry gauze and a bandage or adhesive dressing
- this outer dressing can be re-moistened, allowing the dressing to continue releasing silver ions for several days.
Burn dressings after 48 hours
After 48 hours, the silver dressing is removed and an assessment of the burn injury is made. In the authors’ burn service, silver dressings are not routinely continued after 48 hours. Although silver dressings are toxic to bacteria, there is some in vitro evidence that they inhibit keratinocytes and fibroblasts, which could potentially prolong healing times.7,8 An exception to this would be if the burn presented late to the clinician and appeared to be infected or critically colonised (eg green appearance suggesting Pseudomonas). In this instance, a further 48 hours of nanocrystalline silver would be applied.
Dressings that can be used after this time are summarised below with indications for each. Other dressings are available and all can be sourced online or via other purchasing agreements but prices will vary.
- Hydrocolloids (eg Duoderm [15 x 15 cm thin/thick]; Granuflex)
- crosslinked adhesive dressings of gelatin, pectin and carboxymethylcellulose
- good for low/moderate exudating burns – contacts and holds exudate as a gel inside the dressing
- appropriate for all burn depths
- water repellent and conformable
- use thin hydrocolloids for paediatric dressings but thick hydrocolloids for adults
- NB offensive exudate can be mistaken for infection
- Foams (eg Allevyn [silver 10 x 10 cm]; Biatain [silver 10 x 10 cm]; Mepilex [silver 10 x 10 cm; plain 10 x 10 cm])
- highly absorbent foam dressings suitable for highly exudating burns
- available in plain and silver
- absorb exudate well and therefore help to manage and prevent hypergranulation and maceration
- reduce requirement for dressing changes due to wet dressings
- Alginates (eg Algisite M [10 x 10 cm]; Kaltostat)
- highly absorbent, biodegradable dressings derived from seaweed and contains calcium
- use on moist granulating tissue or small superficial dermal areas of burn
- good for haemostasis if fragile/superficial bleeding
- good for moderately-to-highly exudative sloughy wounds – becomes a gel on contact with wound exudate
- useful under a retention dressing such as Fixomull or Hypafix to secure (in the authors’ experience, direct application of retention dressings to burns can traumatise newly healed epidermis and be difficult and uncomfortable to remove)
- need to soak with water or oil to remove alginates
- retention dressings can be removed using soaking with water or adhesive removal sprays such as Niltac or Appeel
- Hydrogels (eg Intrasite [8 g]; Hydrosorb [10 x 10 cm])
- hydrophilic interactive dressings with very high water content
- able to donate water to the burn and rehydrate dry eschar or necrotic slough and absorb exudate also
- good for dry or sloughy burns, which need some debridement
- good for all burn depths but especially mid-dermal to deeper burns
- no harm to granulation tissue or epithelialisation.
Post-healing dressing/wound care
All burns in the early phase of healing require moisturiser and sun protection. The newly healed epidermis is dry and can have increased melanocyte activity if exposed to sunlight following a burn injury,9,10 causing permanent hyperpigmentation. Current advice in the authors’ burns service is to use sun protection factor 50 (SPF50) sun block and avoid prolonged direct sunlight for two years.
The length of time a conservatively managed burn takes to heal has an impact on scar formation. Burns that take longer than 21 days to heal do so with exaggerated inflammation and have a high rate of hypertrophic scarring.11 This is obviously related to the depth of the burn, as superficial burns heal quickly and deeper burns more slowly. Additional patient factors such as pigmented skin types and personal/family history of poor scarring may affect this risk. These patients may require referral to a local occupational therapy service for scar management with silicone and pressure garments, or to a burns service for consideration of scar revision by laser or surgery.
Conclusion
Smaller burn injuries can be managed well in the community with good wound care and appropriate dressings. The authors’ burns service recommends blister debridement followed by 48 hours of nanocrystalline silver dressings before a decision regarding burn depth is made. At 48 hours, management of injuries that appear large or deep should be discussed with a regional burns service, whereas smaller, more superficial injuries can be dressed with hydrocolloid, foam, hydrogel or alginate dressings and reviewed every two to three days. Certain burn wounds and patients are at higher risk of poor scarring, and these should be identified early to allow scar management to commence.
Every intervention from burn to healing has an impact on the eventual outcome. By managing all burn injuries effectively at every step, we can reduce burn injury morbidity as a community.
Author
Helen E Douglas MBChB, MSc, MD, FRCS (Plast), Burns Fellow, State Burns Service, Murdoch, WA. Helen.douglas@health.wa.gov.au
Fiona Wood FRACS, AM, Head of State Burns Service, State Burns Service, Murdoch, WA
Competing interests: None.
Provenance and peer review: Commissioned, externally peer reviewed.
Acknowledgements
We would like to acknowledge the Wound Healing Institute Australia (WHIA) for their kind permission to use the image of Jackson’s wound model from their Burn module.