Overactive bladder syndrome (OAB) is a common medical condition that has a significant impact on the quality of life in women.1 It is commonly seen in isolation or together with genuine stress incontinence in perimenopausal and menopausal women.1 It is characterised by increased urinary frequency, urgency (with or without involuntary loss of urine) and nocturia.1
OAB is defined by the International Continence Society’s (ICS’s) Standardization of terminology report as ‘urgency with or without urge incontinence, usually with frequency and nocturia’ without evidence of infection or any other proven pathology.1 The prevalence of OAB in 2008 was 10.7% globally, and this is expected to increase to 20.1% by 2018.2 It occurs with equal frequency in women and men, but the severity of symptoms is worse in women. Furthermore, the incidence of OAB increases with age.3 Urge urinary incontinence is a subset of OAB that affects approximately 33% of people with OAB, and is classified as ‘OAB wet’, whereas those who have the symptoms but do not wet themselves are categorised as ‘OAB dry’.1
OAB in any form is a troubling symptom that causes significant distress to patients.1 By contrast, those with pure urinary stress incontinence, which is characterised by incontinence induced by increased pressure in the abdomen secondary to effort or exertion, do not suffer from increased urgency, frequency, or nocturia. Urge incontinence causes more distress to patients than does stress incontinence. Those with both OAB and stress urinary incontinence are concurrently described as having ‘mixed urinary incontinence’.
The multidimensional impact that OAB has on patients’ lives include psychological fear of lack of bladder control, embarrassment and being a burden to others.1 Social aspects of their lives are also disrupted because the symptoms of OAB limit social activity to within safe proximity of a toilet for the fear of urinary leakage.1 OAB also restrains the patient from being sexually intimate and participating in any other forms of physical activities because of the frequent urge to urinate. Patients’ reliance on disposable pads to cope with OAB is costly, adding to another burden to patients and carers.
Apart from specific non-pharmacological treatments that have variable responses, the mainstay of current pharmacological treatment involves the use of muscarinic receptor antagonists. The overall therapeutic effectiveness of muscarinic receptor antagonists is limited by a combination of limited efficacy and troublesome side effects.4 Muscarinic receptor antagonists that are commonly used include oxybutynin, tolterodine, trospium, darifenacin and solifenacin.5
This study used network meta-analysis to evaluate the efficacy, tolerability and costs of commonly prescribed medications involved in managing women with OAB, with or without urinary incontinence. Conventional approaches to meta-analysis are adopted to compare the clinical efficacy and tolerability of drugs used for the treatment of OAB in women. A network meta-analysis was proposed as it allows multiple treatment modalities of different clinical studies to be pitched against one another. Patients with mixed symptoms and urinary stress incontinence were not part of our study interest.
Methods
Eligible randomised controlled trials were included to allow us to evaluate efficacy and tolerability of the assessed medications. A literature search was carried out using two online databases (PubMed and Cochrane). Only studies between 31 July 2000 and 31 July 2015 were included. Summaries of the relevant articles (n = 81) were downloaded and selected on the basis of the inclusion and exclusion criteria. Inclusion criteria encompassed randomised controlled trials, prospective cohort studies and adult women with clinically proven OAB symptoms. Exclusion criteria included case reports and female patients under 18 years of age. Full-text articles (n = 21) were retrieved for eligibility.
Extracted data from eligible studies that were included in quantitative synthesis (n = 5) were then entered into the NetMetaXL software, which offers a graphical output of comparison between the various treatments and also provides a ranking between them.6,7
With multiple drug interventions for OAB delivering similar outcomes, network meta-analysis (also commonly known as a multiple treatment comparison or mixed treatment meta-analyses) provides a variety of ways to visualise and analyse data between interventions that had never been run against each other in a head-to-head trial. This is made possible because the analysis draws strength from indirect evidence to affirm various treatment interventions as though they have been compared with each other directly. As with conventional meta-analysis, network meta-analysis also applies the fixed or random effects models. Therefore, by applying these models, different interventional arms, either with direct or indirect evidence, are compared and rankings to which intervention is superior may be generated and presented as a ranking table or forest plot.
Results
All the trials that were included in our study were randomised trials and of parallel design. Drug therapy was implemented for at least 12 weeks. Four of the trials were placebo-controlled and one was active controlled (Table 1). The placebo arm contained 1688 patients, while all other intervention arms contained a total of 3668 patients.
Figure 1 shows the network of trials with circle ‘A’ representing ‘placebo’ group. Table 2 compares all the drugs against each other in terms of efficacy in treating OAB in a league table, with the left-most being most effective and the right-most being least effective for OAB in women. The values in each cell in the table represent the odds ratio (OR) and, in brackets, the 95% confidence interval (95% CI). An OR of 1 represents equivalent effect. Values in bold indicates significant differences (P <0.05).
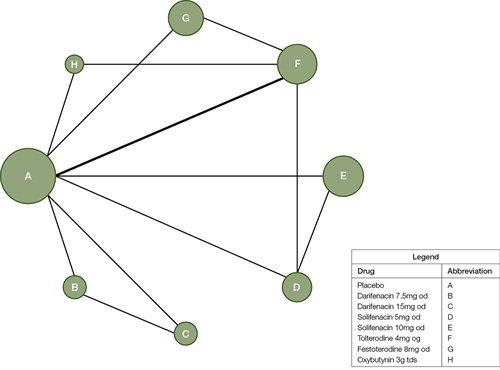
Figure 1. Diagram of the network of trials with each circle representing each intervention arm
The size of the circle correlates with the sample size of the corresponding arm while lines connecting circles highlight that there is direct comparison the two interventions. Thickness of each line is related to the number of patients being directly compared between two arms.
From the league table generated, solifenacin 10 mg once daily (OD) seems to be the most effective in treating women with OAB, followed by oxybutynin 3 mg three times daily (TDS). On the other hand, tolterodine 4 mg OD appears least effective, although it was still significantly more useful when compared with the placebo.
Pooled data from the same sources were compared for the number of adverse events experienced by patients. The adverse events were limited to common side effects of these drugs. For example, dry mouth, dry eyes, blurred vision and constipation are all attributable to the anticholinergic properties of the medications themselves.
Table 3 shows a comparison of every single intervention against each other in terms of adverse events, with the left-most intervention having the least adverse events and the right-most with the most adverse events. The values in each cell within the table represent the OR and, in brackets, the 95% CI. An OR of 1 represents equivalent effect. Values in bold indicate significant differences (P <0.05).
Excluding the placebo, darifenacin 7.5 mg OD had the fewest adverse events, whereas solifenacin 10 mg OD caused the most adverse events when compared with every other intervention except oxybutynin 3 mg TDS.
Table 48 summarises the cost of drugs. Oxybutynin is the cheapest, and darifenacin 7.5 mg, darifenacin 15 mg, fesoterodine 8 mg and solifenacin 5 mg are close behind with similar prices for treatment per month. The most expensive medication is solifenacin 10 mg.
Table 1. Summary of commonly used drugs for the treatment of overactive bladder10–14
|
Trial
|
Dosage
|
Number of female patients
|
Trial length (weeks)
|
Number of patients who responded well to therapy
|
Number of patients who experienced adverse effects
|
Number of patients in arm
|
Criteria for adequate treatment
|
|---|
- Chapple et al (2005)
|
|
900
|
12
|
|
|
|
≥3 dry days/week
|
|
Darifenacin
|
7.5 mg OD
|
|
|
158
|
92
|
288
|
|
|
Darifenacin
|
15 mg OD
|
|
|
171
|
123
|
281
|
|
|
Placebo
|
|
|
|
159
|
59
|
331
|
|
- Chapple et al (2006)
|
|
2,266
|
12
|
|
|
|
>50% reduction in urgency episodes
|
|
Solifenacin
|
5 mg OD
|
|
|
267
|
88
|
431
|
|
Solifenacin
|
10 mg OD
|
|
|
606
|
418
|
916
|
|
|
Placebo
|
|
|
|
402
|
86
|
919
|
|
- Ho et al (2010)
|
|
50
|
12
|
|
|
|
Physician assessment of treatment benefit with ‘much improvement’
|
|
Solifenacin
|
5 mg OD
|
|
|
7
|
10
|
26
|
|
Tolterodine
|
4 mg OD
|
|
|
2
|
6
|
24
|
- Herschorn et al (2009)
|
|
1,697
|
12
|
|
|
|
UPS score showing ‘improvement’
|
|
Tolterodine
|
4 mg OD
|
|
|
273
|
189
|
684
|
|
Fesoterodine
|
8 mg OD
|
|
|
312
|
292
|
679
|
|
|
Placebo
|
|
|
|
120
|
44
|
334
|
|
- Homma et al (2003)
|
|
423
|
12
|
|
|
|
Patient perception of ‘much benefit’
|
|
Tolterodine
|
4 mg OD
|
|
|
68
|
70
|
162
|
|
Oxybutynin
|
3 mg TDS
|
|
|
93
|
117
|
177
|
|
|
Placebo
|
|
|
|
21
|
12
|
84
|
|
|
Total patients
|
|
5,336
|
|
|
|
|
|
|
Total patients in placebo arm
|
1,668
|
|
|
|
|
|
|
|
Total patients in intervention arm
|
3,668
|
|
|
|
|
|
|
Table 2. League table of drugs – Comparison of efficacy in OAB ‘OR (95% CI)’
|
Solifenacin 10 mg OD
|
|
|
|
|
|
|
|
|---|
|
1.09(0.68–1.70)
|
Oxybutynin 3 mg TDS
|
|
|
|
|
|
|
|
1.18(0.93–1.48)
|
1.09(0.69–1.77)
|
Solifenacin 5 mg OD
|
|
|
|
|
|
|
1.50(1.01–2.17)
|
1.38(0.81–2.38)
|
1.27(0.84–1.85)
|
Darifenacin 15 mg OD
|
|
|
|
|
|
1.58(1.15–2.14)
|
1.45(0.94–2.28)
|
1.34(0.95–1.85)
|
1.05(0.70–1.60)
|
Fesoterodine8 mg OD
|
|
|
|
|
1.91(1.33–2.70)
|
1.76(1.02–3.01)
|
1.62(1.08–2.34)
|
1.28(0.91–1.79)
|
1.22(0.81–1.79)
|
Darifenacin7.5 mg OD
|
|
|
|
1.96(1.43–2.61)
|
1.81(1.2 –2.65)
|
1.65(1.19–2.26)
|
1.31(0.86–1.97)
|
1.24(1.00–1.51)
|
1.02(0.69–1.53)
|
Tolterodine 4 mg OD
|
|
|
2.53(2.10–3.05)
|
2.33(1.53–3.63)
|
2.14(1.72–2.67)
|
1.69(1.22–2.36)
|
1.60(1.26–2.06)
|
1.32(0.97–1.82)
|
1.29(1.03–1.66)
|
Placebo
|
Table 3. League table – Comparison of adverse effects among each drug’s ‘OR (95% CI)’
|
Placebo
|
|
|
|
|
|
|
|---|
|
0.47(0.33–0.66)
|
Darifenacin 7.5 mg OD
|
|
|
|
|
|
|
|
0.38(0.28–0.52)
|
0.81(0.51–1.29)
|
Solifenacin 5 mg OD
|
|
|
|
|
|
|
0.36(0.27–0.48)
|
0.77(0.48–1.20)
|
0.94(0.63–1.41)
|
Tolterodine 4 mg OD
|
|
|
|
|
|
0.28(0.20–0.40)
|
0.60(0.44–0.84)
|
0.74(0.46–1.17)
|
0.78(0.49–1.29)
|
Darifenacin 15 mg OD
|
|
|
|
|
0.19(0.13–0.25)
|
0.40(0.24–0.63)
|
0.49(0.32–0.74)
|
0.52(0.41–0.64)
|
0.66(0.40–1.06)
|
Fesoterodine 8 mg OD
|
|
|
|
0.12(0.08–0.19)
|
0.27(0.15–0.47)
|
0.33(0.18–0.56)
|
0.34(0.22–0.52)
|
0.44(0.24–0.78)
|
0.66(0.41–1.06)
|
Oxybutynin 3mg TDS
|
|
|
0.12(0.09–0.16)
|
0.26(0.16–0.39)
|
0.31(0.24–0.41)
|
0.33(0.23–0.49)
|
0.42(0.27–0.65)
|
0.64(0.44–0.95)
|
0.96(0.57–1.66)
|
Solifenacin 10 mg OD
|
|
Bolded figures represent significant difference at 95% CI
|
Table 4. Drugs used to treat overactive bladder*
|
Drug name
|
Strength
|
Cost per tablet (AUD)
|
Cost per day (AUD)
|
Cost per 28 days (AUD)
|
|---|
|
Darifenacin
|
7.5 mg
|
1.71
|
1.71
|
47.80
|
|
Darifenacin
|
15 mg
|
1.71
|
1.71
|
47.80
|
|
Fesoterodine
|
8 mg
|
1.73
|
1.73
|
48.35
|
|
Oxybutynin
|
3 mg
|
0.56
|
1.69
|
47.26
|
|
Solifenacin
|
5 mg
|
1.73
|
1.73
|
48.33
|
|
Solifenacin
|
10 mg
|
2.25
|
2.25
|
62.86
|
|
Tolterodine
|
4 mg
|
1.73
|
1.73
|
48.35
|
|
*Australian dollars converted from British pounds based on prevailing rates at March 2016
|
Discussion
This review compares commonly prescribed medications for the treatment of OAB in women. It answers the research question of whether network meta-analysis is a better means of representing the tolerability, efficacy and cost of commonly used drugs in treating OAB. The use of network meta-analysis presents a unique means of comparing the efficacy and tolerability of medication used to treat OAB in women, compared with conventional approaches. It allows researchers to evaluate the relative efficacy between all interventions, even though head-to-head comparisons have never been carried out.7 Furthermore, it yields definitive and reliable results as it borrows strength from indirect evidence to gain increased reliability between all treatment comparisons.7
Despite the increasing popularity of network meta-analysis, its method and interpretation are poorly understood.7 The risk of bias and strength of evidence from each study is accumulated in the network; therefore, meticulous selection of studies, detection of heterogeneity and its interpretation should be done with great care.7 Several limitations in this analysis are discussed below.
A systematic review by Kakar et al compared solifenacin, tolterodine, oxybutynin and fesoterodine, and concluded that fesoterodine 8 mg OD was the drug to be recommended over other drugs in view of its efficacy and tolerability.9 The drug with the highest efficacy and adverse effect was oxybutynin 5 mg TDS.9 It should be noted that in their study, they only included solifenacin 5 mg OD and not solifenacin 10 mg OD.9
When compared with our results, solifenacin 10 mg OD appears to be more efficacious, while tolterodine 4 mg and darifenacin 7.5 mg are lowest in ranking. Considering the absence of solifenacin 10 mg OD in the study by Kakar et al, our results concur with theirs; that is, oxybutynin is the most efficacious, yet it is ridden with the most adverse effects even though the dose analysed in our study was only 3 mg.
However, when both efficacy and tolerability were considered, solifenacin 5 mg OD appears to be the drug of choice, placing third in efficacy and tolerability in our study. Fesoterodine 8 mg OD, as suggested as the drug of choice by Kakar et al, is not significantly inferior to solifenacin 5 mg OD in our study. Despite that, it appears to have significantly more adverse effects in our analysis. This discrepancy may be attributed to the different methods of analysis, one being a systematic review while the other a network meta-analysis. On the other hand, solifenacin 10 mg OD and oxybutynin 3 mg TDS were more efficacious in comparison with solifenacin 5 mg OD in our study, but these drugs appear to be associated with more adverse events. Our findings were generally in agreement with findings by Kakar et al, which further validates the potential of the network meta-analysis methodology.
Furthermore, when considering the cost of medication (Table 4),8 the price per month’s supply (28 days) of solifenacin 5 mg OD is comparable to most other drugs, the cheapest being oxybutynin 3 mg TDS. Our analysis appears to favour solifenacin 5 mg OD as the drug of choice in the treatment of OAB in view of its efficacy, tolerability and cost. On that account, if solifenacin 5 mg OD fails as first-line treatment, then oxybutynin 3 mg TDS may be considered as it offers similar efficacy to solifenacin 10 mg OD (solifenacin 10 mg OD versus oxybutynin 3 mg TDS; OR: 1.09; 95% CI: 0.68–1.70). A similar adverse effect profile is seen with oxybutynin 3 mg TDS and solifenacin 10 mg OD but at a significantly lower cost.
Study limitations
Given the scarcity of literature that fulfilled the criteria set in this study, the results derived may not constitute the experiences of clinicians and their own views. Only two databases were adopted in this study and, therefore, other studies that might have fulfilled our inclusion criteria may have been left out.
Furthermore, there were difficulties when extracting data with similar endpoints, which was the greatest challenge during this study. Most studies have different endpoints for the treatment of OAB, while the data published for similar endpoints in the form of continuous data lacked variability in the types of drugs studied. In order to standardise endpoints for different studies, which includes a myriad of different drugs used in the treatment of OAB, the researchers took studies that considered patients ‘to be adequately treated’ as a common endpoint for comparison. Although each of the studies consider patients meeting certain criteria to be adequately treated, these criteria vary. Therefore, in the absence of identical endpoints, the results of the study would be less reliable and our results should be interpreted with caution.
Further standardisation of OAB treatment endpoints and well-defined treatment goals will certainly aid in patient management. This will also aid in generation of future data for easier and more unbiased research results.
Conclusion
OAB is a disease of significant burden to women, and pharmacotherapy remains the mainstay of treatment. Using network meta-analysis, we found a useful method of representing the responses of different drugs used in treating OAB. Solifenacin 10 mg OD appears to be the most effective drug, but it causes the most adverse events. The lower dose of the same drug, solifenacin 5 mg OD, appears to be the favoured drug when both efficacy and tolerability are taken into account and it is comparable in cost with most other drugs.
Implications for general practice
OAB is a common urological disorder in women, and GPs can initially manage the symptoms. This includes lifestyle changes, bladder training, pelvic floor exercises and muscarinic antagonists. Failure of these warrants referral to a urogynaecologist or urologist.
Since muscarinic antagonists play an important role in OAB management, this study provides an insight to GPs in comparing efficacy, tolerability and cost of medications, thus aiding them in providing the patient with a more comprehensive management plan.
Acknowledgement
This study was approved by the Joint Committee of Ethics and Research, International Medical University Malaysia. A small grant was provided by the IMU for purchase of scientific articles.
Authors
Sivalingam Nalliah MBBS, MRCOG, FRCOG, FAMM, MCGP, MEd, Professor, Department of Obstetrics and Gynecology, Clinical School, International Medical University Malaysia. Sivalingam_nalliah@imu.edu.my
Pou Wee Gan MPharm, BCPS, 4th year medical student, Clinical School, International Medical University Malaysia, Locum Pharmacist (Guardian Pharmacy (M))
Premjit K Masten Singh, 4th year medical student, Clinical School, International Medical University Malaysia
Piravin Naidu, 4th year medical student, Clinical School, International Medical University Malaysia
Vivian Lim, 4th year medical student, Clinical School, International Medical University Malaysia
Arshad ASA Ahamed, 4th year medical student, Clinical School, International Medical University Malaysia
Competing interests: None.
Provenance and peer review: Not commissioned, externally peer reviewed.