The contraceptive implant, available in Australia as Implanon NXT, is a highly effective (99.95%),1 convenient and rapidly reversible long-acting method of contraception. The single rod (4 cm) implant contains 68 mg of the progestogen etonogestrel, which is released continuously over three years.2 It is listed on the Pharmaceutical Benefits Scheme (PBS), has few contraindications, and has a higher rate of continuation than the pill and injection.3–5 Because women need to return for implant removal, clinicians may have a perception that the discontinuation rate is high. This contrasts with contraceptive methods such as the pill, which women may stop without consultation and result in lost opportunity for timely contraceptive advice.
Implanon NXT training
Implant insertion and removal are within the expertise of all general practitioners (GPs). The manufacturer provides training for doctors (to enquire visit www.implanonnxt.com.au). Most medical indemnifiers require this training for GPs inserting and removing the implants. Simulation practice is available through pharmaceutical representatives or educational providers such as family planning organisations. Some GPs will be more procedurally confident after accessing simulation and/or clinical supervision prior to performing the procedures independently. Increasing numbers of practice nurses are receiving training in implant procedures. Contact your local family planning organisation for information about training for registered nurses and midwives.
Suitable patient
The implant is suitable for women of all reproductive ages, including those who are breastfeeding and immediately postpartum or post-abortion.
There are few contraindications to the use of the contraceptive implant, the only absolute contraindication being current breast cancer. Conditions where the risks outweigh the benefits of the implant include past breast cancer, severe decompensated cirrhosis, hepatocellular adenoma, malignant liver tumours, or where a woman develops ischaemic heart disease, stroke or transient ischaemic attack while using Implanon NXT. It is an option for women for whom oestrogen is contraindicated (eg women with a history of venous thromboembolic disease or migraine with aura).6
Medications that induce liver enzymes interact with Implanon NXT, resulting in an unacceptable risk of unintended pregnancy; an alternative contraceptive method is advised.7 These medications include carbamazepine, phenytoin, rifampicin, ritonavir, nevirapine and St John’s wort.
Benefits and side effects
The implant provides highly effective contraception that is cost-effective over time. Dysmenorrhea improves in around 85% of women.2
Local reactions are uncommon,8 but scarring may occur. Acne may improve, develop or worsen. Other hormonal side effects have been reported, such as headaches, mood changes, weight gain, breast tenderness, loss of libido and abdominal pain; however, evidence of direct causation is limited.6 The implant does not cause a significant reduction in bone density.9
It is important to advise women that their bleeding pattern will change, and about expected bleeding patterns (approximately one in five women have amenorrhoea, three in five have infrequent or irregular bleeding, and one in five have frequent or prolonged bleeding). Approximately half of the women with frequent or prolonged bleeding will improve after three months.10 Explaining these bleeding changes, including an explanation that amenorrhoea is safe and convenient, may improve continuation for some women. Refer to Box 1 for management of troublesome bleeding with the implant.11
Box 1. Management for troublesome bleeding on the implant
- Actively encourage women to return for review of troublesome bleeding
- Management of troublesome bleeding requires exclusion of other causes such as infection, interacting medications and gynaecological pathology. A balance between over-investigation and under-investigation is needed given that altered bleeding patterns are an expected effect of the implant. If there is no suspicion of another cause (eg negative chlamydia, cervical screening up-to-date, no interacting medications, bleeding not associated with other symptoms) reassure the patient that bleeding is within expected limits and not harmful
- If no contraindications, offer the combined hormonal contraceptive pill continuously or cyclically for a three-month trial, which may then be continued concurrently for the duration of the implant use if desired. Other options include a five-day course of either a non-steroidal anti-inflammatory drug (NSAID) such as mefenamic acid 500 mg bd-tds or the antifibrinolytic tranexamic acid 500 mg bd, which can be repeated as required in order to self-manage bleeding episodes
|
Box 2. Quick starting Implanon NXT
- Consider the need for a pregnancy test
- If negative, inform the woman that an early pregnancy cannot be excluded if unprotected intercourse has occurred in the previous three weeks, but that there are no known effects on a pregnancy
- Can be inserted immediately after levonorgestrel emergency contraception, but should wait for five days after use of ulipristal acetate emergency contraception
- Initiate method if desired and feasible
- Advise condom use or abstinence for seven days while waiting for the method to become effective
- Organise a urine pregnancy test (at home or in clinic) in four weeks with reminder/recall
|
When to insert?
Traditionally, and according to the product information, the implant is inserted on days 1–5 of the cycle (day 1 being the first day of menstrual bleeding) when pregnancy is excluded, and is effective immediately. However, this can limit timely access, resulting in the chance of unintended pregnancy while waiting to have the implant inserted. It is acceptable to insert it at other times using an accepted off-label practice known as ‘quick start’.12
When an implant is inserted using quick start, it will not be effective for the first seven days. An early pre-existing pregnancy may not be confidently excluded by a negative pregnancy test if intercourse occurred without reliable contraception in the previous three weeks. Limited evidence from studies and extensive post-marketing surveillance does not indicate an increased risk of teratogenesis if a pregnancy is inadvertently exposed to etonogestrel. Informed consent and clear instructions are required to:
- avoid unprotected intercourse for seven days after insertion
- carry out a urine pregnancy test in four weeks (in the clinic or at home).
The main risk is an undiagnosed pregnancy because of amenorrhoea or spotting being falsely attributed to the effects of the implant. Family planning organisations in Australia have extensive experience with quick start, demonstrating the feasibility and benefits of the method (Box 2).
Consent forms
A consent form for implant procedures may be useful (www.racgp.org.au/download/Documents/PracticeSupport/201105implanonchecklist.pdf). A consent form for quick start is also available (www.fpv.org.au/assets/resources/Quick-start-consent-form.pdf).
Implant procedures
The manufacturer’s training advice should be followed for implant procedures. The following information is not comprehensive, but highlights important and commonly encountered aspects of the procedures.
Setting up for insertion and/or removal
Ensure that there is adequate time, a good light source and complete set of equipment. Some women will feel anxious about the procedure, and may benefit from reassurance and distraction. Position the woman lying on the examination bed with her arm flexed and externally rotated, and her hand next to her head. Ideally, the clinician is seated to facilitate visualisation of the needle during insertion.8
Local anaesthetic tips
It can be efficient to infiltrate with local anaesthetic before setting up the rest of the equipment, re-washing hands and donning sterile gloves. To minimise pain, a local anaesthetic can be administered, using a small gauge long needle (eg 25G 1.5”) and a small syringe (eg 3 mL or 5 mL). The local anaesthetic should be at room temperature when administered.13 Inject slowly, aspirating to avoid intravascular injection. Allow at least a few minutes for the local anaesthetic to work and check for absence of a sharp sensation prior to insertion. Reassure the woman that she is likely to continue to feel pressure sensation after local anaesthetic. If there is insufficient numbness to sharp sensation after 2 minutes, it is worth waiting another 1–2 minutes before considering if further local anaesthetic is needed. Use extreme caution to avoid intravascular injection for women with Wolff–Parkinson–White and Stokes–Adams syndromes.
For insertions:
- Lignocaine hydrochloride 1% 2 mL is recommended,14 but the use of 3–4 mL is standard practice. Some practitioners use lignocaine with adrenaline as the vasoconstriction may reduce bleeding and bruising. The addition of adrenaline increases the risk of cardiotoxic complications and incurs an extra cost.
- Mark the insertion point at 8–10 cm above the medial epicondyle of the non-dominant upper arm and another point around 4 cm above as a direction guide. Use a surgical marker or pen, but avoid puncturing the skin directly through the mark.
- New advice from the manufacturer is to avoid insertion along the sulcus between the biceps and triceps, and to place the implant over the triceps, to avoid the extremely rare complication of intravascular placement.
- Inject the local anaesthetic for a 4 cm length.
For removals:
- Lignocaine hydrochloride 1% with adrenaline (1:100,000) 0.5–1 mL is recommended.
- Mark the distal (elbow) end for incision. It can be helpful to mark the proximal end as pushing here will elevate the distal end of the implant.
- Inject local anaesthetic under the distal end of the implant to avoid swelling over the implant (Figure 1).
- Insertion tips
- Hold the applicator at the textured surface to provide improved control.
- Check the implant is inside the needle after sliding off the cap.
- Pierce the skin at an angle of <30° and then re-angle to a horizontal position to insert the implant superficially in the subdermis. Tent the skin up slightly as you progress the needle to ensure superficial insertion. While sliding the needle subdermally, there may be a sensation of resistance halfway through the insertion as the skin pushes up the lever that secures the implant into the needle.8
- Be careful not to touch the purple slider before getting ready to activate the device as this can result in partial expulsion of the implant.
- After insertion, palpate the implant and, after applying a waterproof dressing, ask the woman to palpate the implant herself before applying the pressure bandage.
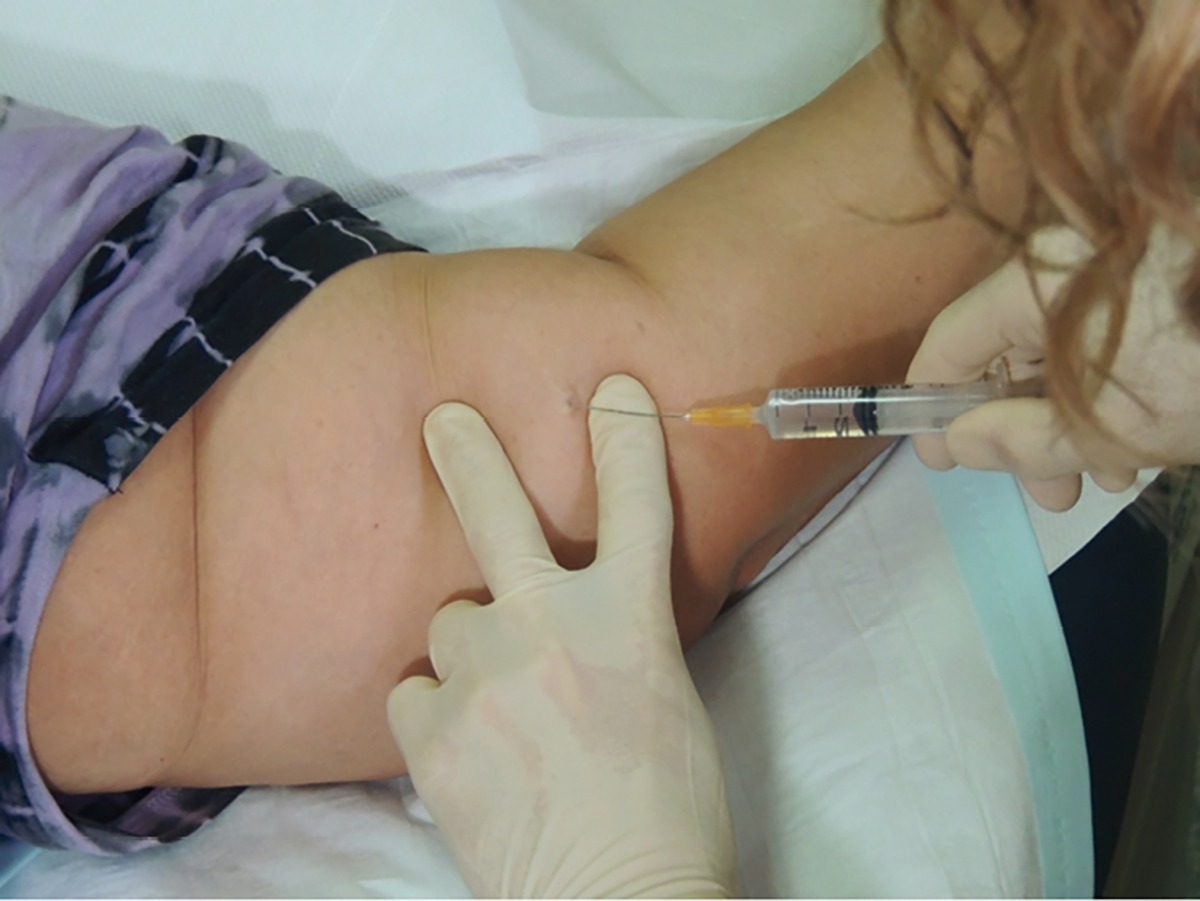
Figure 1. Local anaesthetic for implant removal: injecting under tip
Insertion risks
Non-compliance with instructions can lead to deep and impalpable implants. Deep insertion risks neurological and vascular damage, implant migration and, rarely, intravascular insertion.15
Significant implant site reactions are uncommon, although bruising can be expected. Use hand hygiene, sterile gloves and correct skin disinfection to avoid infection.
Removal timing
The implant should be removed or replaced at three years. If replacement occurs at or prior to the expiry date, there is no need for additional contraceptive precautions.
If the implant has expired, a history is taken to ascertain the risk of pregnancy since the expiry date. If a woman wishes to have a new implant inserted, this can be initiated using quick start.
Removal tips
Make sure the implant can be felt adequately prior to initiating removal.
Assistance in anchoring the proximal end of the implant may be helpful for some clinicians.
- The direction of incision should be parallel to the implant (not perpendicular) and the small incision should be made directly at the distal end (nearest the elbow) of the implant. A common technique is to perform a ‘stab’ incision at about 30°, with the cutting blade turned upwards. While cutting upwards, the tip of the implant is felt with the blade. This technique assists in cutting the capsule around the implant with the initial incision (Figure 2).
- To remove the implant, push on its proximal end to direct the distal end out of the incision (Figure 3). If the implant cannot be visualised, use blunt dissection with forceps to locate the distal end of the implant. Grasp the implant with forceps. In most cases, there will be a fibrous capsule that requires dissection using the blade – transfer the forceps to the non-dominant hand and use the scalpel with the dominant hand (Figure 4).
- Dress with adhesive strips, waterproof dressing and a pressure bandage.
- Advise the woman that the contraceptive effect wears off immediately.
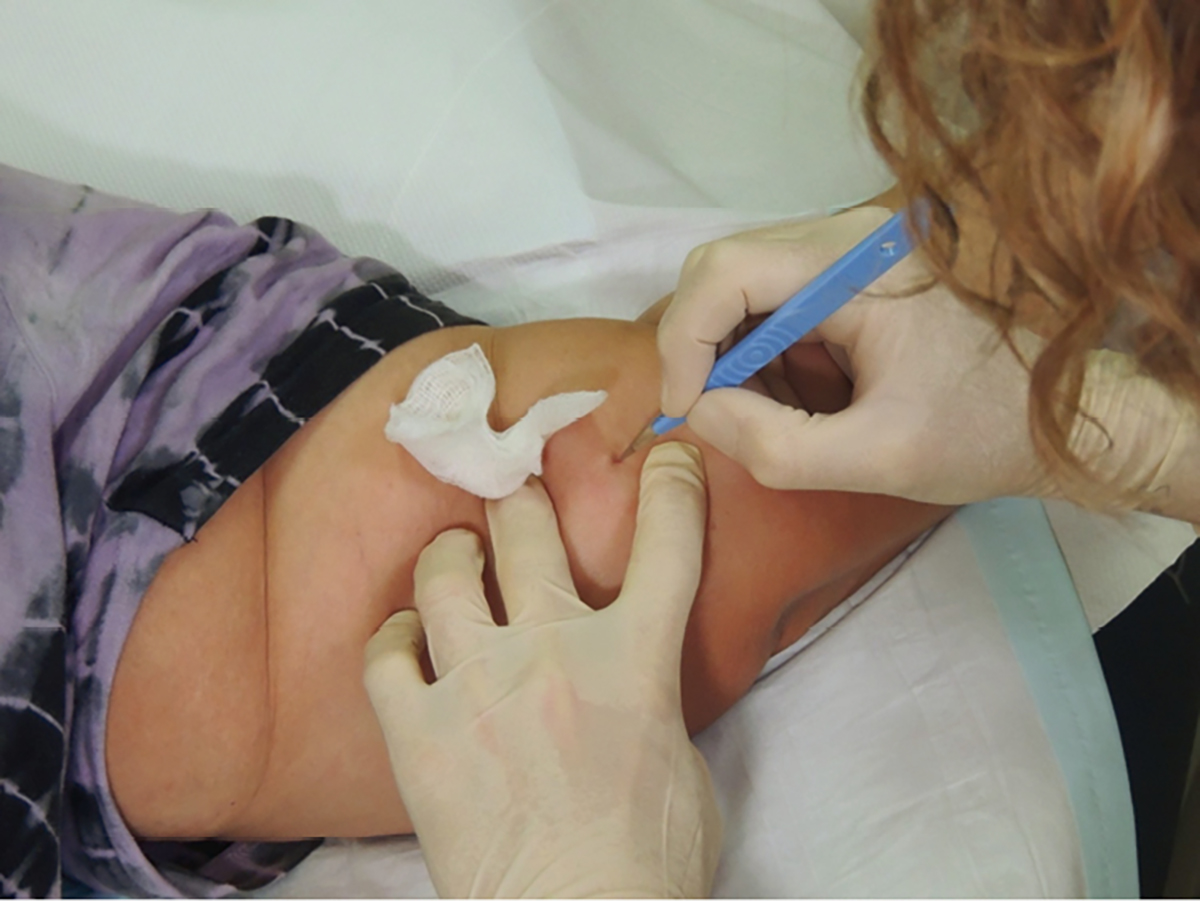
Figure 2. Implant removal: stab incision
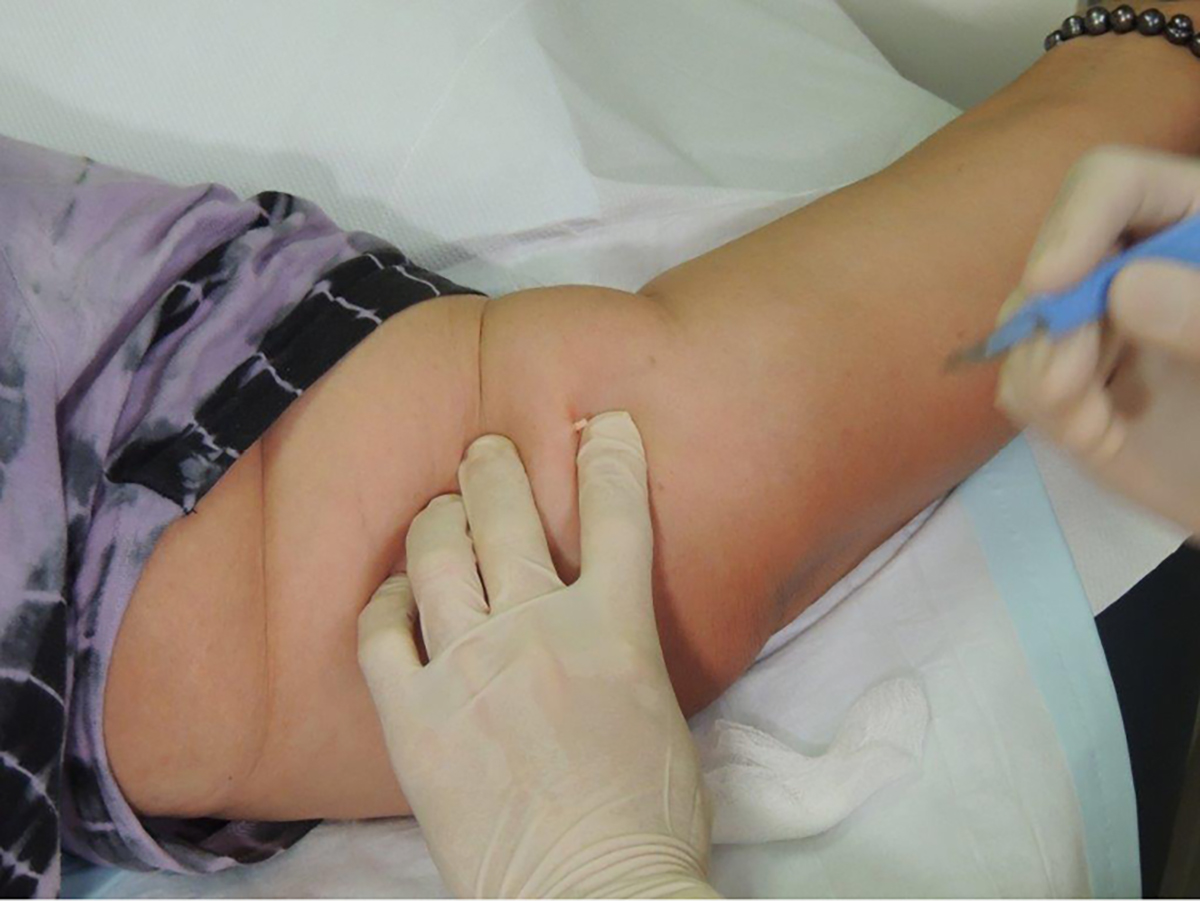
Figure 3. Implant removal: tip exposed and protruding
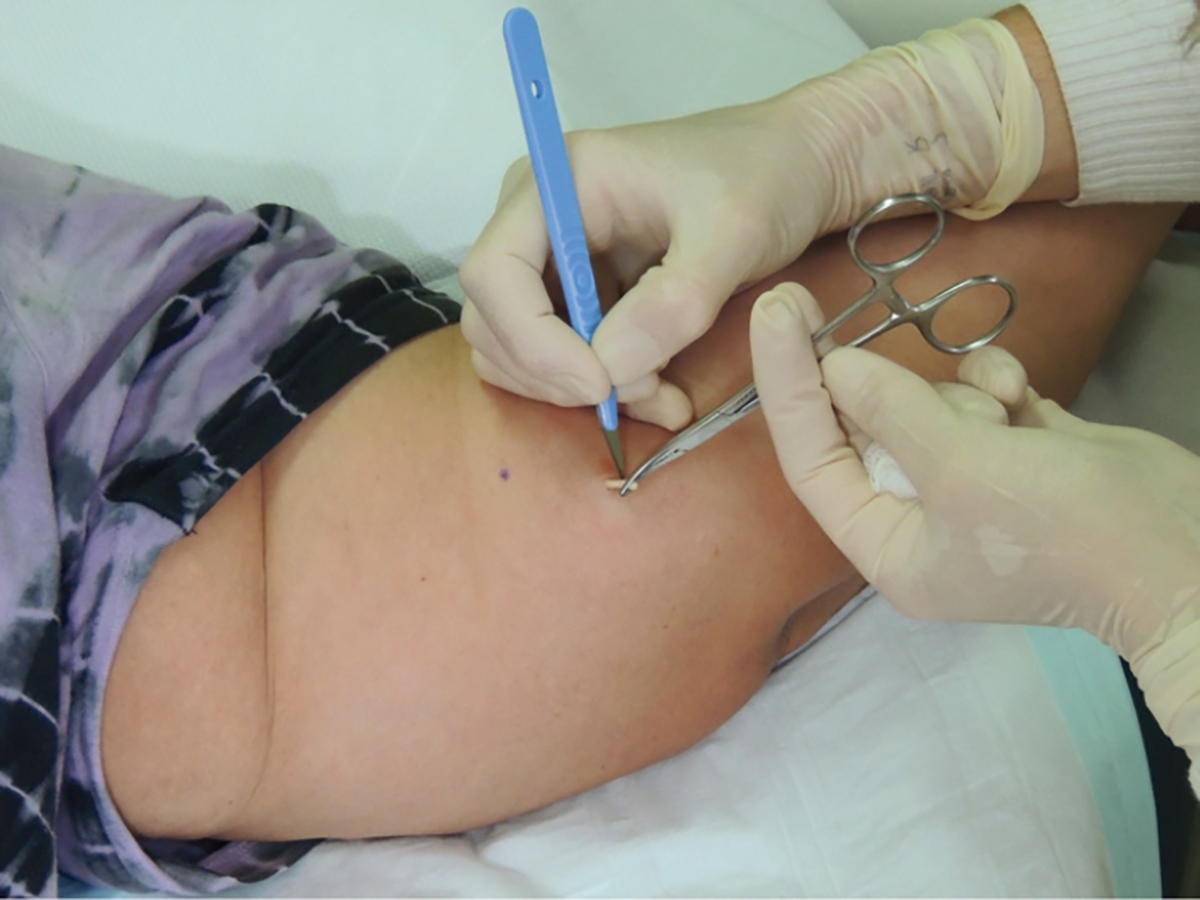
Figure 4. Implant removal: dissecting the fibrous capsule
Deeply placed implants: Difficult to palpate or impalpable
If an implant that is due for removal is difficult to palpate, clinicians who are not experienced in deep removals should refer the patient to a specialised service. Potential removal-related risks include neurovascular damage. An unsuccessful attempt to remove an implant may make subsequent removals more complex procedures because of swelling and scarring.
If an implant is impalpable, removal should not be attempted; carry out a pregnancy test and discuss the need for additional contraception until the implant is located by ultrasonography or X-ray.6 If the deeply placed implant is found to be intramuscular, it should be removed because of the risk of migration. Refer to a local hospital, or phone the state/territory family planning organisation or the manufacturer for advice if required.
Bent or broken implants
Bent implants are likely to continue to be effective. Broken implants are uncommon, and should be referred to an experienced practitioner for removal.6 It is important that all pieces are removed (measuring 4 cm in total length).
Removal and reinsertion tips
If an implant is being replaced, perform a removal and insert the new implant into the incision created for removal.
- Be careful to fully insert the needle to avoid the implant protruding through the incision.
- If a woman has had multiple re-insertions, the implant may gradually be positioned higher in the arm. In this situation, it may be preferable to insert the new implant in a new lower location.
- The manufacturer’s instructions advise injection of local anaesthetic under the distal end of the implant, removal of the implant, then injection of local anaesthetic for 4 cm in the insertion plane prior to re-insertion. However, it can be efficient to insert local anaesthetic along the full length of the implant prior to performing the removal for more experienced clinicians who are able to manage the potential for additional oedema created by the local anaesthetic infusion.
Information to provide after implant procedures
After insertion and re-insertion, provide the woman with the card that comes in the pack and clear post-insertion instructions, including when the method will become effective. Advise the date for removal for the woman to record. Consider the use of a practice software reminder at three years.
For all implant procedures, give advice to keep the site dry and clean for three to five days and to expect some bruising. Some redness, swelling and local pain may occur but are usually minor.8
Medicare Benefits Schedule item numbers
Procedural Medicare Benefits Schedule (MBS) item numbers for Implanon NXT are 14206 (hormone or living tissue implantation by cannula) and 30062 (etonogestrel subcutaneous implant, removal of, as an independent procedure). Both MBS items can be claimed for removal/re-insertion. An attendance item may be applicable for consultation that is independent of the procedure(s).
Alternative insertion sites and contraceptive implants available in other countries
Placement of the implant at alternative sites should be undertaken with caution.16 See the Family Planning Alliance Australia position statement at http://familyplanningallianceaustralia.org.au/larc
If a contraceptive implant was inserted in another country, check carefully for the number of rods. A two-rod, five-year implant called Jadelle is available in New Zealand.17
Resources
Implant factsheets, phone advice and contraception education are available on family planning websites (http://familyplanningallianceaustralia.org.au/services)
Long-acting reversible contraceptives (LARC) information, including efficacy card and guidance for bleeding on progestogen-only LARC card (http://familyplanningallianceaustralia.org.au/larc)
Family Planning NSW, Family Planning Victoria, Family Planning Queensland. Contraception: An Australian clinical practice handbook. 4th edn. Ashfield, NSW: FPNSW, FPV, FPQ, 2016.
Authors
Suzanne Pearson MBBS (Hons), FRACGP, GradCert Clin Teach, Senior Medical Education Officer, Family Planning Victoria, Box Hill, Vic. spearson@fpv.org.au
Mary Stewart MBBS, DFSRH, MPH (Health Promotion), Senior Medical Officer, Research and Education, Family Planning NSW, Ashfield, NSW
Deborah Bateson MA, MSc, MBBS, Medical Director, Family Planning NSW, Ashfield, NSW; Discipline of Obstetrics, Gynaecology and Neonatology, Central Clinical School, University of Sydney, NSW
Competing interests: All authors have attended advisory committees and presented educational forums for Merck Sharp & Dohme (Australia) Pty Ltd. They not been personally financially remunerated for these services. MSD is the sponsor of the contraceptive implant in Australia. Family Planning Victoria and Family Planning NSW receive sponsorship from MSD for some educational courses.
Provenance and peer review: Commissioned, externally peer reviewed.