A 2013 report into diabetic eye disease in Australia found that approximately one million adults are affected by diabetes, and this number is expected to double by 2025. The report also estimates that almost all patients with type 1 diabetes, and more than 60% of those with type 2 diabetes, will develop diabetic eye disease within 20 years of diagnosis.1 One in four Australians with diabetes will be diagnosed with diabetic retinopathy; however, early detection and prompt treatment can prevent up to 98% of visual impairment.2 The 2008 National Health and Medical Research Council’s (NHMRC’s) Guidelines for the management of diabetic retinopathy (Guidelines) recommends regular ocular review of patients with diabetes.3 However, estimates indicate that less than 50% of Australians achieve appropriate screening.4
In partnership with Queensland Health, The Royal Australian and New Zealand College of Ophthalmologists (RANZCO) and Optimed, we completed an NHMRC-funded, open-controlled trial of diabetic screening in general practice using non-mydriatic retinal cameras. The trial included in-depth interviews conducted with practice staff involved in screening for diabetic retinopathy.
The screening outcomes of our trial were published in a separate paper.5 In summary, these outcomes demonstrated a screening rate for diabetic retinopathy of 99% of eligible patients in the intervention practices, compared with 33% in the matched controls. Appropriate follow-up (≤12 months as per the Guidelines) of mild-to-moderate diabetic retinopathy was at 100% in intervention practices, compared with 0–57% in those practices undertaking routine care.5 These findings subsequently informed a Medical Services Advisory Committee (MSAC) submission on screening for diabetic retinopathy in general practice, ultimately resulting in the 2016 release of two new Medicare Benefits Schedule (MBS) items for diabetic retinopathy screening in general practices (Table 1).6 However, there is little evidence‑based information to guide practices in successfully implementing this screening.
This article reports the results of a process evaluation of the open-controlled trial. It seeks to identify and describe the common elements associated with effective screening for diabetic retinopathy, with the overall aim of developing a framework of the key enablers of, and risks to, successful diabetic retinopathy screening in general practice.
Table 1. Group D1 – Miscellaneous diagnostic procedures and investigations
Subgroup 10 – Other diagnostic procedures and investigations
|
MBS item number descriptor 12325*
|
|---|
|
Assessment of visual acuity and bilateral retinal photography with a non mydriatic retinal camera, including analysis and reporting of the images for initial or repeat assessment for presence or absence of diabetic retinopathy, in a patient with medically diagnosed diabetes, if:
(a) the patient is of Aboriginal and Torres Strait Islander descent; and
(b) the assessment is performed by the medical practitioner (other than an optometrist or ophthalmologist) providing the primary glycaemic management of the patient’s diabetes; and
(c) this item and item 12326 have not applied to the patient in the preceding 12 months; and
(d) the patient does not have:
(i) an existing diagnosis of diabetic retinopathy; or
(ii) visual acuity of less than 6/12 in either eye; or
(iii) a difference of more than 2 lines of vision between the 2 eyes at the time of presentation
Fee: $50.00 Benefit: 75% = $37.50; 85% = $42.50
|
|
MBS item number descriptor 12326*
|
|---|
|
Assessment of visual acuity and bilateral retinal photography with a non-mydriatic retinal camera, including analysis and reporting of the images for initial or repeat assessment for presence or absence of diabetic retinopathy, in a patient with medically diagnosed diabetes, if:
(a) the assessment is performed by the medical practitioner (other than an optometrist or ophthalmologist) providing the primary glycaemic management of the patient’s diabetes; and
(b) this item and item 12325 have not applied to the patient in the preceding 24 months; and
(c) the patient does not have:
(i) an existing diagnosis of diabetic retinopathy; or
(ii) visual acuity of less than 6/12 in either eye; or
(iii) a difference of more than 2 lines of vision between the 2 eyes at the time of presentation
Fee: $50.00 Benefit: 75% = $37.50; 85% = $42.50
|
|
*D1.19. Retinal photography with a non-mydriatic retinal camera
This service is separated into two items, MBS item number 12325 and 12326, in line with National Health and Medical Research Council (NHMRC) guidelines’ recommended frequency of repeat testing in persons of Aboriginal and Torres Strait Islander descent and the general population.
This item is intended for the provision of retinal photography with a non-mydriatic retinal camera. Mydriasis is permitted if adequate photographs cannot be obtained through an undilated pupil.
Presenting distance vision means unaided distance vision or the vision obtained with the current spectacles or contact lenses, if normally worn for distance vision.
Detection of any diabetic retinopathy should be followed by referral to an optometrist or ophthalmologist in accordance with the NHMRC guidelines.
Where images are inadequate quality for detection of diabetic retinopathy, referral to an optometrist or ophthalmologist for further assessment is indicated.
Reproduced from MBS Online. Medicare Benefits Schedule, www9.health.gov.au/mbs/search.cfm?q=12325&sopt=S
|
Methods
The open-controlled trial was conducted in 10 general practices (five intervention and five control practices) throughout Queensland, Australia, between February 2011 and February 2014. Figure 1 provides a graphical representation of the trial design. The full trial protocol has been described previously.7
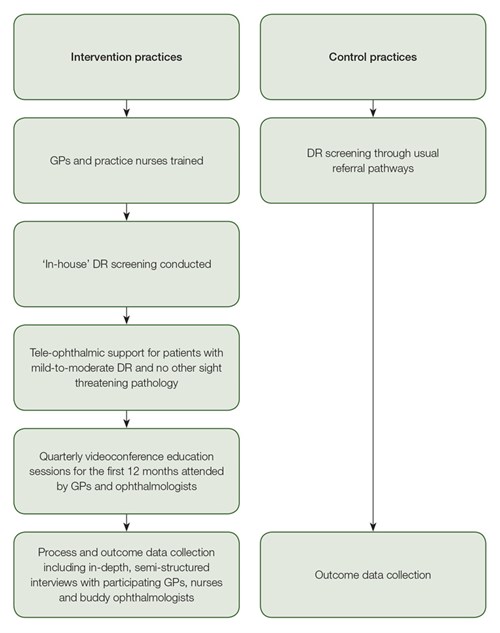
Figure 1. Study processes for intervention and control practices DR, diabetic retinopathy; GP, general practitioner
Intervention practices represented a range of metropolitan, large rural and other rural locations classified using the Rural Remote and Metropolitan Area (RRMA) index.8 Intervention practices had at least 45 patients with type 2 diabetes who were receiving regular diabetes care, and were partnered with a distant ophthalmologist for the duration of the study. Each practice received a non-mydriatic camera, fully installed, and staff training. Participating general practitioners (GPs) in the intervention practices completed a four-hour online diabetic retinopathy upskilling program through the University of Queensland’s Masters of Medicine (General Practice) program, followed by an accreditation assessment through the RANZCO’s Queensland Faculty.7 All patients attending intervention practices for diabetes annual cycle of care assessment were offered screening for diabetic retinopathy, performed ‘in-house’. Patients without diabetic retinopathy were re-screened at a later date according to NHMRC’s Guidelines.3
In-depth, semi-structured interviews were then conducted with practice staff involved in diabetic retinopathy screening from each of the five participating intervention practices. A purposeful sampling strategy was used to provide a rich and detailed overview of health professionals’ experiences of undertaking diabetic retinopathy screening and monitoring. Health professionals represented the range of geographic locations and practice staff involved in the screening (eg GPs, practice nurses, diabetes educators, non-clinical staff). An interview schedule guided discussions, and interviews were undertaken between November and December 2013. All interviews were recorded (either by hand or via digital recording, where possible), transcribed and coded by one member of the research team (LC) using QSR N Vivo software.9 Thematic analysis was used to identify and classify recurrent themes. The study was approved by the University of Queensland’s Behavioural and Social Science Ethical Review Committee (reference number: 2010000584).7
Results
A total of 20 interviews were completed, which comprised 18 in-depth, semi‑structured (recorded) interviews and two informal (unrecorded) interviews. Recorded interviews were held with seven GPs (reviewing images, recording results, managing patients), seven practice nurses, one diabetes educator (photographers, visual acuity, ensuring up-to-date recorded glycated haemoglobin [HbA1C] and blood pressure measures), and three buddy ophthalmologists. Informal interviews were completed with two non-clinical staff members (photographers). The practice staff interviews investigated the models of screening that each practice developed. All interviews explored the perceived benefits, risks and enablers of successful implementation of diabetic retinopathy screening and monitoring. Table 2 provides a summary of three models of diabetic retinopathy screening that were identified, and an overview of the practices and interviewees from each of these models.
Seven themes that related to a combination of enablers and potential risks of the successful implementation of diabetic retinopathy screening were identified:
- up-to-date and accurate diabetes registers
- need for a ‘diabetic retinopathy champion’ in the practice
- appropriate infrastructure and workforce
- opportunities for continuing professional development and patient education
- ability to detect other pathology and conditions
- reduced patient travel
- management and maintenance of communication with all GP partners and other external screening providers (where these existed).
Theme 1: Up-to-date and accurate diabetes register
The problems associated with poor diabetes register (eg inability to easily identify patients eligible for screening and recall) plagued the early phase of the study for the intervention practices and required the commitment of staff to address.
You have to have a decent diabetes register, it has to be up to date … [it’s projects like this] that make you do it and then you realise how out of date it’s got, how messy and how great it is when it’s all sorted out. – Practice nurse, large rural centre
You need a register that’s up to date – it affects everything and you’ve got to have someone on that constantly. – GP, metropolitan centre
Theme 2: Diabetic retinopathy screening ‘champion’ in practice
There was a recognised need for someone to take responsibility for diabetic retinopathy screening in practice for it to become a part of ongoing practice workflow. This was not necessarily seen as the domain of the GP but might also be the practice nurse.
Someone has to take the role of driving it – might be a GP or a nurse … someone, but you need someone take responsibility for it … they need to champion it inside and outside the practice. – GP, large rural centre
Theme 3: Appropriate infrastructure and workforce
This theme related to the need for dedicated and appropriate space to conduct screening, including the space for the patients and development of effective recall processes. In addition, there was a recognised need to have a ‘whole-of-practice’ approach to ensure the implementation of efficient screening processes.
There’s a need to be sure that you have … the space and ability to set aside a room for screening … the volume of patients coming in for screening – make sure you can recall those that need to be – it’s just organisation and … allocating staff and space dedicated to it. – GP, large rural centre
I take the photos but … staff on the front desk [have to] tell the patients they were going to be here a bit longer … everyone has to know what’s going on and what they need to do. – Practice nurse, large rural centre
Table 2. Diabetic retinopathy screening practices and interviewees
|
Practices undertaking diabetic retinopathy screening
|
|---|
| |
Practice A
|
Practice B
|
Practice C
|
Practice D
|
Practice E
|
|
RRMA score*
|
4
|
1
|
1
|
3
|
3
|
|
Screening model†
|
DRS1
|
DRS2
|
DRS3
|
DRS3
|
DRS3
|
|
Number of accredited GP image reviewers (n)
|
1
|
2
|
1
|
1
|
2
|
|
Number and role of photographer(s) (n)
|
1 diabetes educator
|
2 practice nurses
|
2 practice nurses
|
2 non-medical staff
2 nurses
|
1 nurse
|
|
Ophthalmologists
|
1
|
1
|
1
|
1
|
1
|
|
*RRMA scores: 1, Metropolitan; 2, Other metropolitan; 3, Large rural centre; 4, Small rural centre; 5, Other rural centre
†Models of DR screening used in practices:
DRS1 = Dedicated diabetic retinopathy screening clinic; appointments made for patients and images reviewed at the time, with the patients involvement
DRS2 = Opportunistic diabetic retinopathy screening; images reviewed in bulk by accredited GP and patients recalled if necessary
DRS3 = Dedicated diabetic retinopathy screening clinic; appointments made for patients; images reviewed in bulk and patients recalled later if necessary
|
Theme 4: Opportunities for continuing professional development and patient education
The engagement in diabetic retinopathy screening led to opportunities for continuing professional development. This, in turn, led to continual improvements in the management of patients with diabetic retinopathy.
It’s continual learning, you talk to the ophthalmologist about the different or stranger cases … and its working in an area I’m really interested in … so that’s a bonus. – GP, rural centre
We got really good relationships with our ophthalmologist and developed better management plans for patients and you do actually learn a lot too – talking to the ophthalmologist. – GP, metropolitan centre
It’s great to be able to work with the GPs, provide information and support – and do it all over the telephone or on the computer – so you can discuss the images. – Ophthalmologist 1
Following on from this, GPs from rural and regional practices and the support ophthalmologists noted the powerful effect of having an image immediately available to discuss with the patient, and the positive influence of this on patient self-management. This was particularly so when video-conferenced ophthalmic consultations were held between a patient, GP and an online ophthalmologist.
Well … we get the photos in ‘real-time’, so we can use them for patient education in relation to ongoing diabetes management – I can sit here, spin the screen around and show them the image – look at this and this ... its powerful. – GP, rural centre
Having patients there at the time … and discussing the results with them and the GP – that is definite benefit and it’s all done in the practice. – Ophthalmologist 2
Theme 5: Ability to detect other pathology and conditions
GPs noted that the non-mydriatic cameras gave them the ability to identify and investigate other pathology and conditions as part of screening (eg eye injuries, hypertension). It encouraged GPs to explore other benefits of having the cameras.
The use of camera for detecting other pathology – other conditions … things like eye injuries – that’s really useful and value adds to what we can do – I’m sure we’ll find more things as we go on … – GP, small rural centre
We could use the cameras to look at other issues and conditions – a big one was hypertension, especially in our elderly patients … that was a positive spin off … – GP, metropolitan centre
Theme 6: Reduced need for patient travel
GPs and nurses in metropolitan and rural centres noted that the need for patient travel was reduced as a result of the introduction of in-practice diabetic retinopathy screening. In one rural practice, interviewees also noted that the video-conferenced ophthalmic consultations further reduced the need for patient travel.
Reduced need for urban travel for some patients – it makes access to screening easier and therefore they’re more likely to receive it; particularly so for the older patients who can have driving problems in the central city. – Nurse, metropolitan centre
We stopped some of our patients having to travel for screening – the screening and management is all done here in practice – it’s a ‘one-stop shop’. – GP, large rural centre
Theme 7: Management of relationships with GP colleagues and external screening providers
As well as the need for a screening ‘champion’ in the practice, GPs also noted the need to maintain positive relationships with their colleagues. This was to ensure there was no perceived competition between GPs performing the eye image reviews and recording for patients in practice, and those GPs who were not.
It’s difficult – especially if your colleagues don’t understand what you’re doing … we had people here think we were ‘patient poaching’ by doing the screening so we just had to let them know what was going on … We made sure we sent all the patients back to their own GP with the outcomes … – GP, large rural centre
There was also the need to maintain relationships with other local screening providers (eg optometrists). Overall, GPs in rural and regional areas described positive relationships with local optometrists. They wanted to ensure they continued to communicate with these services as they introduced screening for diabetic retinopathy into their own practice workflow. In some instances, there were unexpected benefits relating to the introduction of general–practice based screening for diabetic retinopathy.
We’re careful managing the relationship we have with our local optometrists here – they’re really good and we don’t want to be seen like we’re trying to put them out of business … you have to manage that type of thing … We keep in touch with them and let them know what’s going on and that’s been good. – GP, large rural centre
It’s a small community, so the word gets around … when our local optometrists got to know we had the camera – we started getting records and screening information about our patients sent in that we didn’t get before … so that was unexpected, a good bonus really … we don’t want to compete with anyone … there’s more than enough people needing regular screening here to get through. – GP, small rural centre
Finally, GPs noted the need to have a clear and defined business approach to diabetic retinopathy screening, and to ensure it was embedded into the practice’s way of working.
I mean … you need to have a good, sound business model that will work within your practice. – GP, metropolitan centre
Overall, introducing screening in general practice was a positive experience, summarised by one rural GP, who noted:
At first I was a bit worried – the screening can take extra time … but the nurse took time to learn to take the photos, we got all key things sorted – like the patient register, screening room … and now it’s easy to fit in to the day-to-day routine – it’s just part of what we do. – GP, small rural centre
These themes were summarised into a framework of key components, which practices should be mindful of as part of planning for and undertaking diabetic retinopathy screening and monitoring (Table 3).
Table 3. Enablers and risks of successful diabetic retinopathy (DR) screening and monitoring in general practice
|
Enablers
|
|---|
|
A champion of diabetic retinopathy screening
- A ‘champion’ or ‘driver’ of DR screening within the practice
- Specific recognition and promotion of DR screening to general practitioner colleagues in practice and other local screening providers external to the practice (eg optometrists)
|
|
Appropriate infrastructure and workforce
- Up-to-date, accurate diabetes registers and recording systems prior to commencing screening
- Allocated camera space, teleconsultation options for GPs and partner ophthalmologists
- Dedicated staff to perform photography
|
|
Education and training for staff and patients
- Competence of GPs in diabetic retinopathy image interpretation and recording
- Ongoing education and training on requirements of diabetic retinopathy screening in practice and annual cycle of care
- Use of eye images to educate patients in diabetes self-management and annual screening requirements, where needed
|
|
Active promotion of additional benefits
- Use of the non-mydriatic cameras to detect other pathology and conditions
- Reduced need to travel in order to access diabetic retinopathy screening, for patients in both rural and urban centres
|
|
Risks
|
|---|
No one to manage professional relationships internal and external to the practice
- Lack of dedicated staff to gain expertise in, and take responsibility for, diabetic retinopathy screening in practice
- Perceived competition between GP screener and GP colleagues in practice
- Perceived competition between the screening practice and other local screening providers, such as optometry
|
|
Insufficient time given to developing appropriate infrastructure and workforce
- Incomplete and/or improperly coded diabetes registers
- Incomplete recording systems
- Lack of dedicated space for DR screening
|
|
Lack of education and awareness of practice staff
- Need for GPs to undertake training in image interpretation and the implementation of screening approaches
- Lack of awareness of all staff on the requirements of screening for diabetic retinopathy in practice
|
|
Additional considerations
|
|---|
|
Screening for diabetic retinopathy should:
- Fit within existing practice systems and processes
- Fit within annual cycle of care requirements
- Have a defined business model.
|
Discussion
Our research identified the powerful role general practice can play in addressing the gap between the estimated current (<50%) and recorded screening rates (99%), demonstrated by practices across rural, regional and urban areas that participated in the trial.5,10 Not all practices will wish to deliver this service, but the availability of MBS support now allows these practices to have the option.
This article allows participating practices to identify the key issues involved in that choice on the basis of the elements raised by practice interviewees as key to the successful introduction of a diabetic retinopathy screening service.
Clinician leadership is consistently described in innovation and change management literature as critical to the successful introduction and adoption of new ways of working.11,12 Following from this, it is an all-of-team approach that ensures key elements of the screening service – information and communication, recall, booking, photography, image review, patient involvement and education, and accurate record keeping – are complete and maintained. All these elements are underpinned by accurate and well‑maintained recall databases that maximise screening coverage, avoid inappropriate patient approach, and maximise practice efficiency.
Recently launched education programs now support general practice training in image interpretation and the processes to implement successful models of screening for diabetic retinopathy in a range of geographic practice contexts.13 Finally, patients and their families must understand the risks of asymptomatic diabetic eye disease, and the importance of their GP and optometrist working together to optimise appropriate review. As 65% of Australians with diabetes may require annual screening for diabetic retinopathy to meet NHMRC screening criteria,14 the opportunity for coordinated, alternating general practice and optometry annual review to optimally support patients is significant.
While interviewees identified the recognised benefits that diabetic retinopathy screening in their practices conferred, they noted that the development and implementation of a successful screening approach also required a viable business model. The availability of a $50 MBS item for general practice diabetic retinopathy screening, the chronic disease management nurse item number 10997 and the recent reduction in the cost of cameras now allow larger practices with a significant diabetes population a revenue-stream to support appropriate screening delivery.
Limitations of the study
The limitations of the original diabetic retinopathy screening trial have been detailed elsewhere.1 In relation to the collection and review of qualitative data, it should be noted that the same researcher responsible for the management of the original trial also undertook all in-depth interviews and analysis. This has the subsequent potential for interviewer bias. However, this researcher is highly experienced in qualitative research methods and interview techniques. Interviews were also only conducted with those staff in the intervention practices actively participating in diabetic retinopathy screening and monitoring, and so feedback from other practice staff was not sought.
Conclusion
Screening for diabetic retinopathy has now been found to be effective and acceptable in a range of Australian general practices. It offers improved patient access to screening, particularly for patients in rural and remote areas. Recalling, recording and following assessment over time is key to effective screening for diabetic microvascular disease, and the patient’s general practice or healthcare home is increasingly the appropriate site for this.
The new MBS items for diabetic retinopathy screening have given general practice the opportunity to contribute to preventing blindness in the many Australians with diabetes who are currently missing out on appropriate care. This article allows clinicians and practice teams to understand the enabling infrastructure that underpins effective implementation.
Authors
Lisa Crossland PhD, MPHTM, BA (Hons), Senior Research Fellow, Centre for Health Reform and Integration, Mater Research Institute, Brisbane, Qld. l.crossland1@uq.edu.au
Claire Jackson MBBS, MD, MPH, CertHEcon, GradCert Management, FRACGP, FAICD, Professor in Primary Care Research, Primary Care Unit, The University of Queensland, Herston, Qld.
Competing interests: None.
Provenance and peer review: Not commissioned, externally peer reviewed.
Funding: Funding for the study was provided through an NHMRC Partnership Grant.
Acknowledgements
The authors would particularly like to thank the GPs, practice staff and ophthalmologists who participated in this study.