Over the past decade, bariatric–metabolic surgery has emerged as an attractive, evidence-based option that offers significant and durable weight loss and improved health outcomes for those with clinically severe obesity. Given the exponential increase in the number of Australians with clinically severe obesity over recent decades, and the growing numbers of bariatric–metabolic surgeries performed, primary care physicians increasingly encounter patients who have had, or are considering, bariatric–metabolic surgery. The aim of this article is to provide guidance on:
- evidence for the use of bariatric–metabolic surgery
- selection of appropriate patients for bariatric–metabolic surgery
- pre-operative work-up prior to surgery
- acute and long-term complications after surgery
- long-term lifestyle, nutritional and comorbidity follow-up.
Types of surgery
The three most commonly performed operations for the treatment of obesity in Australia and worldwide are sleeve gastrectomy (SG), adjustable gastric band (AGB) and Roux-en-Y gastric bypass (RYGB; Figure 1). A brief description of these procedures and their key characteristics are summarised in Table 1.
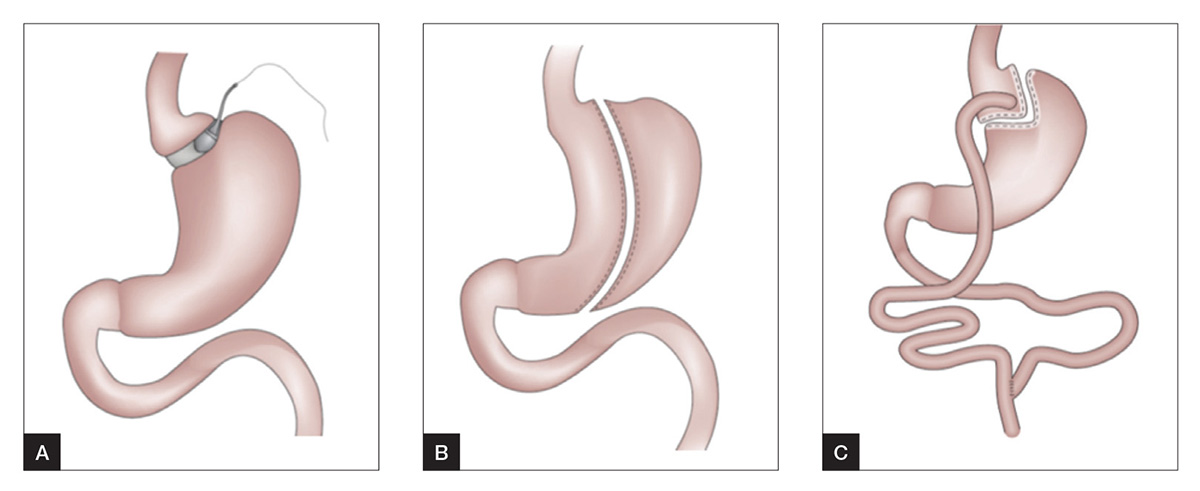
Figure 1. Common bariatric–metabolic procedures3
A. Adjustable gastric band
B. Sleeve gastrectomy
C. Roux-en-Y gastric bypass
Evidence for the use of bariatric–metabolic surgery
Weight loss
A landmark prospective study on bariatric–metabolic surgery, the Swedish Obese Subjects study, has indicated that maximal weight loss of 20–32% was achieved at one to two years after surgery.1 Weight loss was sustained at 18% even after 20 years.2 In general, RYGB offers the largest mean percentage weight loss (25–35%), followed by SG (20–30%) and AGB (17–20%).3 Weight loss seen after AGB is slow and gradual, whereas rapid weight loss can be expected with SG and RYGB. With all three procedures, the weight loss achieved translates to numerous health benefits, including reduced mortality, especially from cardiovascular disease and cancer; improvement in obesity-related diseases; and improved physical functioning and emotional wellbeing.4
Mortality
Large meta-analyses have found that cardiovascular and cancer mortality were approximately halved after bariatric–metabolic surgery when compared with the matched non-surgical cohort5,6 in studies of up to 14 years’ duration.
Improved diabetes control and diabetes remission
Type 2 diabetes mellitus (T2DM), a global epidemic that mirrors the rise in obesity rates, has recently been a major focus of bariatric–metabolic surgery. Marked weight loss from bariatric–metabolic surgery leads to substantial improvement in T2DM, even to the point of remission. Randomised controlled trials have found that treatment of obesity and T2DM with bariatric–metabolic surgery is superior to medical therapy in controlling hyperglycaemia and cardiovascular risk factors over the medium term.7,8
Improvement in other obesity-related complications
Apart from better glycaemic control, sustained weight loss following bariatric–metabolic surgery also results in significant improvement in other obesity-related complications, and some of the key complications are shown in Table 2.
Health-related quality of life
It has been estimated that obesity has a greater negative impact on quality of life than 20 years of ageing, an impact that persists even after accounting for demographics, health habits, medical conditions and depression.9 Numerous studies have consistently found substantial improvement in health-related quality of life following weight loss from bariatric–metabolic surgery.7,9
Table 1. Summary of characteristics of current bariatric–metabolic procedures3,25
| |
Adjustable gastric band
|
Sleeve gastrectomy
|
Roux-en-Y gastric bypass
|
|---|
|
Description
|
Adjustable silicone band placed just below the gastroesophageal junction, applying gentle pressure that suppresses hunger. Level of restriction can be adjusted by varying the amount of fluid placed in the band
|
Greater portion of the fundus and body of the stomach is removed. Gastric volume is reduced by about 80%
|
Combination procedure:
1. Small stomach pouch created, thereby reducing gastric volume
2. The pouch is joined to the jejunum, hence, diverting nutrients from lower stomach, duodenum and proximal jejunum
|
|
Mean total body weight loss
|
17–20%
|
20–30%
|
25–35%
|
|
Mortality rate (at 30 days)20,21
|
0.03–0.1%
|
0.3–0.5%
|
0.1–0.4%
|
|
Morbidity at one year
|
4.6%
|
10.8%
|
14.9%
|
|
Nutritional concerns
|
Low (deficiencies in iron, vitamin B12, folate, thiamine)
|
Moderate (deficiencies in iron, vitamin B12, folate, calcium, vitamin D, thiamine, copper, zinc)
|
Moderate (deficiencies in iron, vitamin B12, folate, calcium, vitamin D, thiamine, copper, zinc)
|
|
Advantages
|
Effective, with good long-term weight maintenance
Degree of restriction adjustable
Reversible
Lowest morbidity and mortality rate
|
Very effective with good mid-term weight maintenance
|
Largest amount of weight loss with good long-term weight maintenance
Highest rate of diabetes remission (for patients with pre-existing type 2 diabetes mellitus)
|
|
Disadvantages and key complications
|
Highest long-term re-operation rate
Gastric pouch dilatation, erosion of band into the stomach, leaks to the adjustable gastric band system, weight regain
|
Staple line leak, gastroesophageal reflux disease, dilatation of the gastric remnant, weight regain
Limited long-term data
|
Staple line leak, dumping, stomal ulcer, intestinal obstruction, gallstones, nutritional deficiency, altered alcohol metabolism, weight regain
|
Patients who could benefit from surgery
The pre-operative assessment starts with a detailed history and physical examination (Box 1). Eligibility criteria for surgery are primarily based on body mass index (BMI) and the presence of obesity-related complications,10,11 including:
- individuals with BMI >40 kg/m2
- individuals with BMI >35 kg/m2 with one or more obesity‑related complications.
In a recent statement by major international diabetes organisations, which was endorsed by the Australian Diabetes Society, bariatric–metabolic surgery was proposed to be an established treatment option in the algorithm to manage T2DM.12 Bariatric–metabolic surgery is recommended for:
- all individuals with T2DM and BMI ≥40 kg/m2
- individuals with BMI 35–40 kg/m2 with inadequate glycaemic control despite lifestyle and optimal medical therapy.
Box 1. Pre-operative assessment for bariatric–metabolic surgery
|
History
Weight history (eg age of onset of obesity, minimum and maximum weight), previous weight loss attempts (including diets, medications, previous weight-loss surgery), triggers for weight gain/regain
Presence of obesity-related comorbidities, family history, medication history
Assessment of current lifestyle: dietary behaviour and physical activity levels; work and home environment, psychosocial support
Physical examination
Weight or body mass index, neck circumference, waist circumference, blood pressure
Signs of specific causes of obesity (eg hypothyroidism, Cushing’s syndrome)
Investigations
Routine laboratory screen: fasting lipids, full blood count, urea and electrolytes, liver function tests, coagulation screen, urinalysis; fasting glucose and glycated haemoglobin (HbA1c) to screen for diabetes
In pre-existing T2DM: C-peptide level to predict diabetes remission
Nutritional screen: iron studies, vitamin B12, folate, 25-OH vitamin D
Gastrointestinal evaluations such as abdominal ultrasound and endoscopy may be required
Electrocardiogram: referral to cardiologist for echocardiogram or stress testing may be required
Polysomnography if moderate or severe obstructive sleep apnoea suspected
Psychological evaluation
Assess commitment, motivation, understanding and expectations of surgery
Psychiatry referral if known or suspected psychiatric illness or substance abuse
Management
Cigarette smokers should stop smoking, preferably at least six weeks prior to surgery
Pre-operative very low energy diet at least two weeks prior to surgery to induce weight loss and reduce complications
|
In addition, bariatric–metabolic surgery may be considered in those with a BMI of 30–35 kg/m2 and with uncontrolled hyperglycaemia, despite optimal medical therapy.11,12 However, the number of studies in this population is limited, and there is a lack of long-term data demonstrating net benefit.
Absolute contraindications in bariatric–metabolic surgery are few and include contraindications to general anaesthesia, serious blood or autoimmune disorders, active drug or alcohol abuse, and severe, untreated psychiatric illness. Patients with limited life expectancy due to cardiopulmonary or other end-organ failure or metastatic/inoperable malignancy are also not suitable for surgery.
Throughout the pre-operative workup process, new information will be obtained, and the risk–benefit profile changes. As with any operation, the potential benefits of surgery must outweigh the peri-operative and long-term risks of surgery.
As a general rule, bariatric–metabolic surgery is strongly recommended and should be prioritised for individuals with super obesity (BMI >50 kg/m2) or class III obesity (BMI >40 kg/m2) with serious complications that are sensitive to weight loss. Surgery is also suitable for younger patients who are likely to develop complications of obesity and subsequent reduced quality of life over time without active intervention. On the other hand, increasing age is a risk factor for postoperative complications and mortality.13,14 The risks of overweight and obesity attenuate with age and the role of intentional weight loss becomes less clear.15 Caution is advised if the patient is older than 65 years of age.
A large observational study found that bariatric–metabolic surgery was associated with reduced risks of gestational diabetes and excessive fetal growth, but with increased risk of pre-term birth and small-for-gestational-age infants.16 All women of childbearing potential should be counselled on contraceptive choices and the need to avoid pregnancy pre-operatively and 12–18 months postoperatively. There is concern that rapid weight loss during the early postoperative period may be detrimental to the developing fetus. Patients who become pregnant after bariatric–metabolic surgery should be monitored closely for appropriate weight gain, nutritional supplementation and fetal wellbeing.
Most importantly, patients must be able, willing and motivated to adhere to the postoperative lifestyle changes, nutritional supplementation and follow-ups that are necessary to ensure safety and success.
Pre-operative assessment
Once a decision has been made for bariatric–metabolic surgery, a series of detailed assessments would be organised (summarised in Box 1).
Nutritional assessment
A state of high-energy malnutrition is often observed in clinically severe obesity, which is masked by ample energy excess. Up to 80% of bariatric–metabolic surgery candidates have micronutrient deficiencies pre-operatively, and appropriate nutritional assessment allows deficiencies to be corrected prior to surgery.17
Table 2. Pre-operative optimisation of obesity-related complications4,26
|
Obesity-related complication
|
Pre-operative screening and optimisation
|
Improvement after weight loss post-surgery
|
|---|
|
Type 2 diabetes mellitus
|
Glycated haemoglobin (HbA1c) and fasting glucose to screen for diabetes
Aim for good glycaemic control (HbA1c <7%) prior to surgery
|
Better glycaemic control and a reduced medication burden
Diabetes remission in some cases
|
|
Cardiovascular disease
|
Electrocardiogram (ECG) and cardiac risk assessment
Referral to cardiology if high cardiovascular risk, presence of cardiac symptoms or abnormal ECG
|
Reduction of cardiovascular morbidity of >50% (compared to body mass index [BMI] and age matched controls)
|
|
Non-alcoholic fatty liver disease
|
Liver function tests
Consider abdominal ultrasound scan if liver function test increased, specifically to detect fibrotic liver disease
|
Improved liver histological appearance
Potential regression of established liver disease
|
|
Obstructive sleep apnoea (OSA) and asthma
|
Screening questionnaire (eg STOP-BANG) to identify those at risk for OSA
Refer to sleep specialist if STOP-BANG score ≥327
|
Significant improvement in apnoea–hypopnoea index
Remission of OSA in some cases
|
Identifying and optimising complications prior to surgery
Pre-operative assessment also includes identification and optimisation of obesity-related complications, with the aim to improve peri-operative safety and outcomes after surgery. Key complications are shown in Table 2.
Psychosocial assessment
Prior to surgery, thorough assessment of the patient’s behaviour, home and work environments, family dynamics, and their ability to incorporate nutritional and lifestyle changes should be conducted. Incorrect beliefs and unrealistic expectations on what the procedure can achieve must be rectified. Depression, anxiety and other psychiatric disorders are prevalent in individuals considering bariatric–metabolic surgery; referral to a psychologist or psychiatrist should be considered if psychiatric illness is suspected.
Anatomical assessment
As part of the pre-operative assessment, evaluation of the upper gastrointestinal anatomy may be performed, depending on factors such as the presence of gastrointestinal symptoms or type of surgery being considered.
Pre-operative preparation
Weight loss prior to bariatric–metabolic surgery can reduce liver volume and visceral adiposity, which may ease technical aspects of the surgery and lead to improved short-term outcomes.18
In T2DM, pre-operative weight loss with medical nutrition therapy can also improve glycaemic control. This can reasonably be achieved using very low energy diets (VLEDs) consisting of meal replacements that provide ≤3350 kJ/day (≤800 kcal/day). VLED is started at least two weeks prior to surgery. During a VLED, patients with T2DM should self-monitor their capillary blood glucose regularly, especially if they are on insulin or insulin secretagogues. Reductions in insulin doses are often necessary while on a VLED to prevent hypoglycaemia.
Smoking is associated with adverse postoperative outcomes, including poor wound healing, anastomotic ulceration and pneumonia. All patients who smoke should be advised to stop smoking, preferably six weeks before surgery. Following RYGB, alcohol metabolism is impaired and high-risk groups should abstain from alcohol consumption to reduce the risk of alcohol-use disorder postoperatively.
Procedure selection
The choice of surgical procedure is guided by the individual’s characteristics, aims of therapy and available surgical expertise. RYGB produces the greatest amount of weight loss and may be appropriate for individuals with a very high BMI. AGB has lower peri-operative risk, compared with RYGB, but higher rate of re-operation for inadequate weight loss19 and thus may be suitable for older individuals or those at lower BMI ranges. RYGB provides the greatest rate of diabetes remission, a consideration for patients with T2DM. RYGB is also the treatment of choice in patients with gastro-oesophageal reflux disease (GORD); SG and AGB may exacerbate GORD, whereas GORD frequently improves after RYGB. Finally, good-quality, postoperative care is crucial to the success of the AGB. The decision for AGB should take into account the availability of an appropriate after-care program.
Acute and chronic complications
Bariatric–metabolic surgery is generally regarded as safe, with low morbidity and mortality that is comparable to elective laparoscopic cholecystectomy.20,21 Procedure-specific complications are shown in Table 1, and the presence of abdominal pain, nausea or vomiting should alert consideration of these key complications as possible differential diagnoses. Presentation of common and important acute and long-term complications after bariatric–metabolic surgery are summarised in Table 3. These features should prompt referral back to surgical or nutritional team.
Postoperative follow-up
Lifestyle changes after surgery
Recommended postoperative lifestyle changes and follow-up care are summarised in Box 2. This care is best delivered by a multidisciplinary team involving the bariatric physician, bariatric surgeon, general practitioner, nurse, dietitian, exercise physiologist and psychologist.
Detailed information on postoperative diet and nutritional recommendations have previously been reviewed elsewhere,4,17 and recommended nutritional supplementation and monitoring are shown in Box 2.
Maintenance of physical activity after bariatric–metabolic surgery aids in maintaining muscle strength, enhanced fitness and greater weight loss. Moderate-intensity physical activity of at least 150 minutes per week and a target of 300 minutes per week including strength training two to three times per week is recommended.11
Table 3. Specific adverse events following bariatric–metabolic surgery and management plan
|
Adverse events
|
Presentation
|
Management
|
|---|
|
Acute complications
|
|
Surgical complications (eg leaks, perforations, obstruction, infection, haemorrhage)
|
Abdominal pain, tachycardia, breathlessness, drop in haemoglobin
|
Usually detected during immediate postoperative period and managed by the surgical team
Presence of these symptoms should prompt urgent referral back to the surgical team
|
|
Hypoglycaemia (usually in patients with pre-existing diabetes)
|
Sweating, dizziness, headaches, palpitations
Low capillary blood glucose on testing
|
Fairly common, especially in patients on insulin or insulin secretagogues
Stop sulphonylureas, and stop insulin or decrease dose
Close self-monitoring of capillary blood glucose
|
|
Dumping syndrome
|
Abdominal pain, diarrhoea, nausea, flushing, palpitations, sweating, agitation, and syncope after meals rich in simple carbohydrates
|
Dietary modification, with small regular meals containing protein and complex carbohydrates
Acarbose may be helpful in some refractory cases
|
|
Long-term complications
|
|
|
|
Iron-deficiency anaemia
|
Microcytic, hypochromic anaemia, lethargy, anorexia, pallor, hair loss, muscle fatigue
|
Oral iron supplements, consider intravenous iron for severe deficiency
Vitamin C to increase iron absorption
Rule out bleeding ulcers, neoplastic disease or diverticular disease
|
|
B12 deficiency
|
Macrocytic anaemia, leukopenia, glossitis, thrombocytopenia, peripheral neuropathy
|
Vitamin B12 repletion (oral or intramuscular)
Prevention – B12 containing multivitamin supplementation
Annual serum B12 level evaluation
|
|
Thiamine deficiency
|
Neurological symptoms, Wernicke’s encephalopathy in severe cases
|
Appropriate postoperative diet, with regular dietitian follow-up
Screen for other nutritional deficiencies
Thiamine supplementation
|
|
Over-restricted gastric band (for patients with adjustable gastric band)
|
Maladaptive eating, gastro-oesophageal reflux disorder, vomiting, regurgitation, chronic cough, or recurrent aspiration pneumonia
|
Reduce amount of fluid in gastric band
Consider referral to bariatric surgeon for assessment of band position and function
|
|
Weight regain
|
Maximal weight loss usually achieved at one to two years after surgery, with some weight regain thereafter
|
For patients with laparoscopic adjustable gastric band – evaluation of band, adjust as required
Consider adjuncts (eg very low energy diet, pharmacotherapy)
Consider referral back to weight management clinic
|
Adjustment of chronic medications
In patients with T2DM, adjustments to the antidiabetes agents are frequently necessary in the early postoperative period to prevent hypoglycaemia. Insulin secretagogues (ie sulphonylureas, metiglinides) should be discontinued and insulin doses reduced as appropriate. Metformin should be continued postoperatively and withdrawal considered if stable non-diabetic glycaemia (ie glycated haemoglobin in the normal range for at least six months) is demonstrated. Screening for diabetes complications should continue even with diabetes remission, at least for the first five years.12
The effect of weight loss on lipids, especially low-density lipoprotein, is variable and generally modest. Therefore, lipid-lowering medications should not be discontinued unless clearly indicated. Although blood pressure often improves with weight loss, the effect is variable and incomplete, and occasionally transient. Therefore, antihypertensives should be actively titrated on follow-up. Medications with a narrow therapeutic index (eg warfarin, digoxin, lithium, antiepileptic medications) also require close monitoring and titration because of altered oral drug bioavailability following bariatric–metabolic surgery.
Cost-effectiveness and access to care
A systematic review of numerous studies has demonstrated that bariatric–metabolic surgery is clinically effective and cost-effective for moderate-to-severe obesity, compared with non-surgical treatment.22 However, access to surgery remains an issue in the Australian public healthcare system. The majority of procedures are still performed in private hospitals, and for those who can afford private health insurance and the associated out-of-pocket costs.23 Meanwhile, obesity and its complications disproportionately affect those at lower socioeconomic status.24 Improving accessibility of surgery to the population most at need remains a key priority, especially as bariatric–metabolic surgery is now widely regarded as less discretionary, but rather a medically necessary treatment option for those with clinically severe obesity.
Conclusion
In properly selected patients with clinically severe obesity, there is clear net benefit in terms of improved health outcomes and reduced mortality following bariatric–metabolic surgery. Given the high prevalence of obesity in Australia and worldwide, GPs are well placed to identify suitable patients who could benefit from bariatric–metabolic surgery. Similarly to other chronic conditions, GPs play a crucial role in the management of obesity and in the multidisciplinary, long-term postoperative support and monitoring that is important for optimal outcomes after surgery.
Box 2. Postoperative follow-up checklist
|
Monitor weight loss progress and complications at each visit
Monitor adherence to appropriate diet and physical activity levels
Medication review –
- Avoid nonsteroidal anti-inflammatory drugs
- Adjust antihypertensives, lipid medications as appropriate. These medications should not be discontinued empirically
- Adjust diabetes medications. Requirement for anti-diabetes medications often decreases, and in many cases, diabetes remission is achieved. Preference for use of agents with favourable weight profile
Nutritional supplements –
- Adult multivitamin and multimineral – containing iron, folic acid, thiamine, vitamin B12. Doses: two daily for sleeve gastrectomy or Roux-en-Y gastric bypass; one daily for adjustable gastric band
- Citrated calcium – elemental calcium 1200–1500 mg/day
- Vitamin D – titrate to 25-OH vitamin D levels >30 ng/mL. Typical dose required 3000 IU/day
- Additional iron and vitamin B12 supplementation as required, based on lab results
Laboratory assessment –
- Full blood count, urea and electrolytes, liver function tests, uric acid, glucose, lipids (every 6–12 months)
- 25-OH vitamin D, iPTH, calcium, albumin, phosphate, B12, folate, iron studies (annually, more frequently if deficiencies identified)
|
Authors
Phong Ching Lee MBChB, MRCP (UK), Consultant Endocrinologist, Obesity and Metabolism Unit, Department of Endocrinology, Singapore General Hospital, Singapore
John Dixon MBBS, PhD, FRACGP, FRCP (Edin), NHMRC Senior Research Fellow; Head of Clinical Obesity Research, Baker Heart and Diabetes Institute; Adjunct Professor, Primary Care Research Unit, Monash University, Vic. john.dixon@bakeridi.edu.au
Competing interests: John Dixon has provided consultancy services to Apollo Endosurgery, Covidien, Nestle Health Science, Bariatric Advantage, I-Nova and Novo-Nordisk. Phong Ching Lee has no competing interests to declare.
Acknowledgement: John Dixon acknowledges the support of the National Health and Medical Research Council (NHMRC) through a senior research fellowship.
Provenance and peer review: Commissioned, externally peer reviewed.