From somewhat humble beginnings as ‘an organism looking for a disease’,1 Clostridium difficile has emerged as a serious worldwide public health threat, capable of causing a range of problems from mild diarrhoea to fulminant colitis and death. The impact of C. difficile infection (CDI) on healthcare systems is a growing international concern. In 2010, the Society of Healthcare Epidemiology of America identified the epidemiology, pathogenesis, treatment and prevention of CDI as one of the five most important clinical challenges facing healthcare epidemiology.2 Recently, the Centers for Disease Control and Prevention (CDC) named C. difficile as the most important antimicrobial-resistant threat to healthcare, requiring ‘urgent and aggressive action’.3
Since the discovery in 1978 that C. difficile causes pseudomembranous colitis, this anaerobic, Gram-positive bacillus has been regarded almost exclusively as a hospital concern, and exogenous acquisition from the hospital environment has been considered the only source of infection. Recently, changes in the epidemiology of CDI have become apparent; not only has there been the emergence of new, highly virulent strains that are capable of causing severe outbreaks in healthcare facilities,4 but infections are now occurring in the community.5 Although C. difficile has always been a cause of diarrhoeal disease in patients presenting to general practice,6 rates of community-associated (CA) CDI have increased. This change has been accompanied by the appearance of severe disease in groups of patients who would otherwise lack ‘classical’ risk factors for CDI, including antibiotic exposure, recent hospitalisation and advanced age. All-cause mortality among CDI cases is high, and advanced age and infecting ribotype are associated with poorer outcomes.7
This article provides a summary of what is currently known about CA CDI and the implications for Australian general practitioners (GPs).
Definitions
Definitions for CA and healthcare-associated (HA) CDI were published in 2007 in order to standardise surveillance.8 Although intended as interim definitions, these terms were widely adopted and categorised laboratory-confirmed CDI cases as shown in Figure 1 (adapted with local terminology). In Australia, there is mandatory reporting of hospital-identified (HI) CDI – cases of CDI (HA and CA) that are identified within any part of the hospital system.
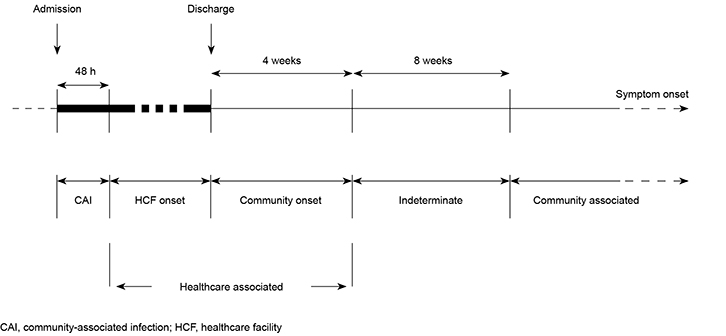 |
Figure 1. Enhanced surveillance timeline for HA or CA CDI definitions8
Adapted with permission from McDonald LC, Coignard B, Dubberke E, et al. Recommendations for surveillance of Clostridium difficile-associated disease. Infect Control Hosp Epidemiol 2007;28:140–45. |
Pathogenesis, treatment and prevention
As is the case with many clostridial infections, disease due to C. difficile is toxin-mediated. C. difficile produces three toxins alone or in combination: toxin A, toxin B and binary toxin, although the role of binary toxin is still being debated. Non-toxigenic strains do not cause disease in humans or animals, and colonisation with these strains may offer protection against symptomatic diseases.9 CDI requires disruption of the colonic flora, most commonly through the use of antibiotics, coupled with exposure to the organism through the faecal–oral route. An overview of CDI pathogenesis is shown in Figure 2.
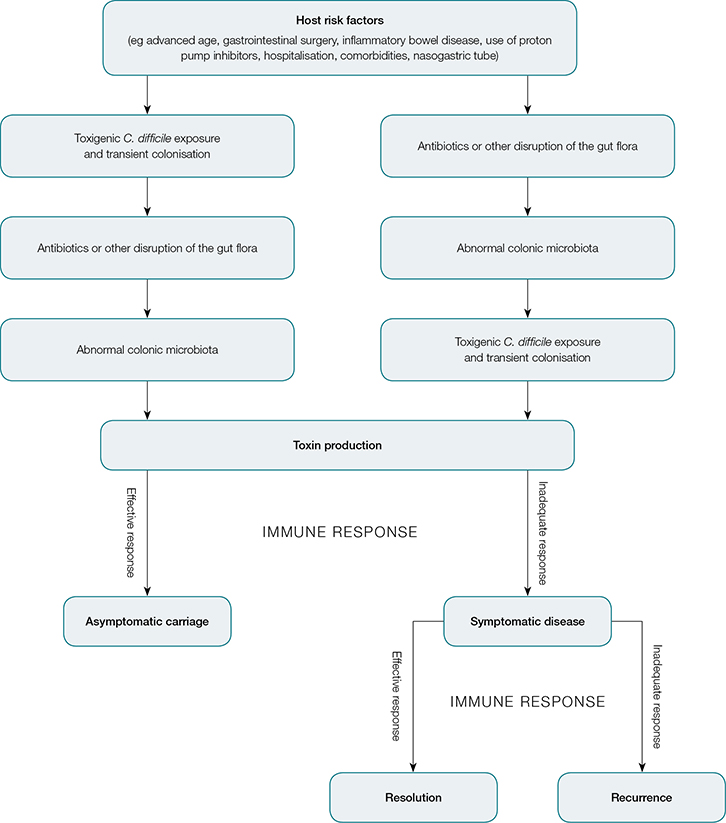 |
Figure 2. Pathogenesis of C. difficile infection34
Adapted with permission from the Massachusetts Society of Medicine from Leffler DA, Lamont JT. Clostridium difficile infection. N Eng J Med 2015;372:1539–48. |
In many cases, withdrawal of the implicated antibiotic will allow re-establishment of the normal microbiota, and this alone will result in disease resolution. In more severe cases, treatment with antibiotics (most commonly metronidazole for mild disease, or vancomycin for more severe or recurrent disease) may be required.10 New treatment modalities, such as fidaxomicin and faecal microbiota transplants, are now being used more widely to treat patients who have recurring symptoms.11 Approximately 20% of patients with CDI experience recurrent disease through relapse or re-infection.11
Asymptomatic, long-term gastrointestinal (GI) tract colonisation by C. difficile is rare, although transient colonisation occurs frequently, reflecting transmission through environmental contamination. People with C. difficile colonisation of the GI tract may then spread the spores into the environment. Infection prevention and control measures for C. difficile in hospitals are usually focused on documented CDI cases showing symptoms of disease.
C. difficile produces spores that can persist on contaminated surfaces for months or years.12 The spores are not eradicated by alcohol-based hand rubs – products that are in wide use and often take the place of traditional hand washing with soap and water if hands are not visibly soiled. The persistence of these spores presents a challenge for effective cleaning and eradication.
Changing epidemiology
Recently, surveillance data on HI CDI in Australia were aggregated for the first time.13 This included all cases of CDI diagnosed in hospital patients from 1 January 2011 to 31 December 2012 in 450 public hospitals in all Australian states and the Australian Capital Territory. Patients admitted to inpatient wards or units in acute public hospitals (including psychiatry, rehabilitation and aged care), as well as those attending emergency departments and outpatient clinics, were included. The annual incidence of HI CDI increased significantly from 3.25 for every 10,000 patient days (PD) in 2011 to 4.03 for every 10,000 PD in 2012. Trends were similar for HA and CA CDI, and a subgroup analysis determined that 26% of cases were CA CDI.
In Western Australia (WA), enhanced surveillance has found a much higher prevalence of CA CDI in younger patients (authors’ unpublished data), in keeping with previous research.5 Current prevalence estimates of CA CDI in WA are sourced from HI cases (ie those identified at an acute care facility, patients presenting to an outpatient appointment, or seen and/or admitted through the emergency department). This group of patients is clearly an under-representation of all CA CDI cases, and detection in general practice is a key missing piece in the overall picture of CA CDI.
Risk factors for CA CDI
In the broadest terms, two events are required for the advent of CA CDI. First, an individual needs to be exposed to an agent that affects the GI tract microflora, or have an underlying GI tract problem such as inflammatory bowel disease (IBD). Second, the individual needs to be exposed to C. difficile, most likely in the form of spores. Sources of C. difficile may be the home, the food chain, water, workplace or environment, as this species is ubiquitous. Currently, these potential sources are of significant interest as identification of risk factors for infection may help in the early diagnosis and subsequent management of CDI.
Antibiotic exposure
Antibiotics and their role in CA CDI have been widely researched. Deshpande et al found an increased risk of CDI across a number of different groups of antibiotics, with clindamycin followed by fluoroquinolones found to pose the greatest risk.14 The risks in the Australian community are likely to be different because of different prescribing practices; however, accurate data are not available. Antimicrobial stewardship has long been considered essential practice in hospitals because of concerns over the emergence of antibiotic resistance, and the risks to public health of prescribing practices in the community are also of concern. In Australian general practice, there was a significant decline in the use of antibiotics from 1998 to 2009, thought to be due, in part, to public health campaigns targeting this issue.15 However, there should be increased awareness of the potential to develop CA CDI in patients receiving antibiotics in the community. This is particularly relevant in high-risk groups such as haematology or oncology patients, who are more susceptible to infection in the community because of their immunocompromised status.
Other inciting factors
It is important to note that antimicrobial exposure is not always a precursor for CDI. A study of nearly 1000 CA CDI cases in the US found 36% of cases had no documented prior use of antibiotics.16 Further, 18% of cases had no outpatient healthcare exposure, and 41% only had low-level outpatient exposure. Coupled with evidence of CA CDI occurring in younger patients, CDI should be considered in patients who lack classical risk factors. Other risk factors that have been described include increasing age, malignant haematological disorders and cytotoxic drugs, non-surgical GI procedures and anti-ulcer medication.17 Exposure to proton pump inhibitors remains controversial, although the original research was conducted using a large general practice database.18 Patients with IBD remain at greater risk of CDI, and recent publications suggest an increased occurrence of severe CDI in these patients.19
C. difficile in animals (human and non-human)
C. difficile colonises the GI tract of many human neonates. An English study found C. difficile in the stools of 71% of infants in a special care nursery, with 94% of strains producing toxin in vitro.20 This study concluded that acquisition from environmental sources (rather than maternal transmission) was the likely source of colonisation, as evidenced from progressive acquisition during the course of hospitalisation.20 Although carriage is common in this group, disease is rare, and neonates are believed to lack the necessary toxin-binding sites in the GI tract. However, neonates and children <2 years of age may transmit this organism to susceptible individuals who come into contact with them. In a study of 57 patients with CA CDI who were diagnosed by their GPs, a significant association between CDI and contact with infants <2 years of age was found.21
C. difficile colonisation and infection have been described in numerous non-human animal species, with documented similarities between human and animal strains.22–24 Further research is required, however, to provide conclusive evidence of the transmission between humans and animals. Potential animal sources include production (food) animals, companion animals and wild animals. In Australia, C. difficile has been found in animals used for food, including sheep, pigs and cattle, but prevalence is much higher in very young animals.25–27 Similarities between human and animal strains, as well as increasing incidence in humans of strains that were formerly exclusively found in animals (eg ribotype 078), suggest inter-species transmission, specifically, unidirectional transfer from animals to humans.22,24
C. difficile in food
The possibility of C. difficile transmission through food has been examined by several authors.28–30 There is evidence of contamination in retail meat as well as vegetable products that may have been indirectly contaminated through fertilisation of the soil with the faeces of infected or colonised animals. Several studies conducted over the past 20 years across numerous countries have detected C. difficile in retail meat products, including beef, veal, chicken, pork, lamb, venison, buffalo, smallgoods and seafood.28–30 There was significant heterogeneity in the prevalence of C. difficile across studies, and some results are suggestive of low levels of contamination; however, without knowing the infectious dose (the number of C. difficile spores that need to be detected in food to present a risk for infection), these results may still be of significance.
Implications for general practice
Gastroenteritis from all causes is the sixth most common new problem managed by Australian GPs, seen at a rate of 1.1 cases for every 100 patient encounters.15 At this rate, the typical GP could expect to see more than 100 cases of new diarrhoeal illness every year. The proportion of these cases with CA CDI is unknown. Current clinical practice guidelines preclude routine laboratory testing for acute episodes of diarrhoeal illness, as most are self-limiting; however, microbiological testing is indicated for severe disease, recent antibiotic use or hospital admission.31 Data from the Netherlands suggest GPs test for C. difficile in only 7% of stool samples and about 40% of these are positive.32 If this is similar in Australia, there will need to be significant change in current clinical practice if CA CDI is to be detected in this setting. Another important issue is laboratory testing for CDI, which remains in a state of flux despite significant improvement over the past few years.10,33
While there is a role for GPs to reduce CA CDI through prescribing practices, there is also an opportunity to diagnose and treat CA CDI in a timely manner. A patient with CDI presenting in the community may not have any classical risk factors; however, antibiotic exposure still represents the most significant risk factor for the development of disease. Patients who develop diarrhoea following antibiotic exposure should be investigated for CDI. Although it may not always be possible to prevent CA CDI, a multi-pronged approach of prudent prescribing, patient education and adequate testing are effective strategies to control this public health threat.
There are several potential reservoirs for CA CDI that need further investigation, including environmental, animal and human sources. There is an increasing incidence and severity of CA CDI, and evidence of disease in populations not traditionally considered ‘at risk’. This suggests that symptomatic patients presenting with no recent history of hospitalisation or antibiotic use should be investigated for potential CDI, in addition to being tested for other potential enteric pathogens. Primary care providers are an integral part in the developing knowledge of CA CDI, with timely diagnosis and management necessary to deliver effective treatment.
Key points
- CDI is a growing concern in the community and severe disease is being diagnosed in groups of patients who lack classical risk factors for CDI – antibiotic exposure, recent hospitalisation and advanced age.
- Exposure to antibiotics remains the most significant inciting factor for developing disease, although it is not always a precursor.
- Certain high-risk groups, such as haematology or oncology patients and patients with IBD, are more susceptible to infection in the community.
- There are several potential reservoirs of C. difficile in the community that need further investigation, including environmental, animal, food and human sources.
- Primary care providers are an integral part in the developing knowledge of CA CDI, with timely diagnosis and management necessary to deliver effective treatment.
Authors
Lauren Tracey BHlthSc, MPH, Programme Officer, Healthcare Associated Infection Unit, Department of Health, Communicable Disease Control Directorate, Shenton Park, WA. lauren.tracey@health.wa.gov.au
Andrew Kirke BSc (Hons), MBBS, FRACGP, FACRRM, Senior Lecturer, Rural Clinical School of WA, University of Western Australia, Bunbury, WA
Paul Armstrong BE (Hons), MBBS, MAppEpid, FRACP, FAFPHM, Director, Department of Health, Communicable Disease Control Directorate, Shenton Park, WA
Thomas V Riley MAppEpid, MAppEpid, PhD, FASM, FAAM, FRCPath, FFSc (RCPA), Professor, Microbiology, University of Western Australia, Nedlands, WA
Competing interests: Thomas Riley’s institution has received consultancy fees from bioMérieux and Otsuka Australia Pharmaceutical Pty Ltd, and grants from Cepheid and Becton, Dickinson and Company.
Provenance and peer review: Commissioned, externally peer reviewed.