Chronic pancreatitis is a slow, irreversible process characterised by pancreatic parenchymal loss, fibrosis and possible calculus formation.1,2 Its principal aetiological factor in Western countries is the consumption of alcohol.3,4 Despite a distinctly different pathogenesis, many cases of acute pancreatitis harbour underlying chronic pancreatitis.5 Thus, effective management is often compromised as the only interface such patients have with the health system is in an acute context, typically consisting of admission to a surgical unit.
Chronic pancreatitis, first and foremost, presents a diagnostic dilemma. If defined in strictly histological terms (as above), the gold standard test would be a pancreatic biopsy; but this is not feasible or safe for diagnostic work-up in clinical practice. The radiological gold standard diagnostic test has been endoscopic retrograde cholangiopancreatography (ERCP),6 but this can no longer be justified for purely diagnostic purposes because of potential risks. Other morphologically based investigations, such as magnetic resonance cholangiopancreatography (MRCP)7 and endoscopic ultrasound (EUS), are better alternatives.8 Various functional tests, largely based on the premise that exocrine insufficiency is a proxy for parenchymal damage, are also possible. The most readily available and practicable of these is the faecal elastase-1 concentration, which requires collection of a random stool sample.9
None of the above morphological or functional diagnostic tests is completely sensitive (‘early’ symptomatic cases may not be detected) or specific (test-positive cases without symptoms may not warrant active management). In clinical practice, therefore, a composite diagnosis based on clinical, morphological and functional criteria is more pragmatic. Various diagnostic algorithms have been proposed;10–12 these may be adapted to local specifications.
Once diagnosis is established, patient management is, ideally, what the French would call la prise-en-charge, approximately translated as ‘taking charge of’. It acknowledges a certain holism where management not only involves therapeutic interventions, but also the diagnostic work-up on which those interventions are contingent, and the necessary iterative adjustments following any intervention. Such a philosophy embodies the desired application of the chronic disease paradigm to pancreatitis. Primary care is ideally placed to provide this.
Initiation of la prise-en-charge in the primary care setting should be guided by symptoms with which patients present. There is no evidence that pre-symptomatic screening is of any benefit, although there are certain provisos (see below). There are four essential clinical scenarios that primary care providers may face, and these should arouse suspicion of underlying chronic pancreatitis. While not necessarily mutually exclusive, each engenders a particular management focus.
Chronic abdominal pain
The most common clinical presentation for chronic pancreatitis is abdominal pain, characteristically epigastric in location with radiation to the back. Two typical patterns of pain have been noted: brief episodes over several days with long remissions, and prolonged periods of persistent pain and/or clusters of severe pain exacerbations.13
Assuming an initial abdominal ultrasound excludes gallstone disease, further diagnostic imaging may include computerised tomography (CT) scan, MRCP, EUS or ERCP – these are not only to confirm the diagnosis of chronic pancreatitis, but also to detect a small proportion of patients whose pain is purely due to the obstruction of the main pancreatic duct by calculi or strictures. Patients with obstruction of the main duct could be referred to a pancreatic surgeon for consideration of a drainage and/or resectional procedure. Distal pancreatic duct strictures may be amenable to endoscopic stenting. On occasion, imaging may also reveal one or more pseudocysts. These alone are unlikely to be the cause of chronic pain, but if one pseudocyst is sufficiently large, the patient may be referred for consideration of an endoscopic drainage procedure. Contingent on any major surgical intervention is the need to definitively eliminate existing risk factors, notably alcohol, utilising whatever psychosocial support measures are available.
For the majority of patients with pancreatitis who experience chronic pain, including those in whom operative interventions have failed, pain is due to inflammatory irritation of parenchymal nerve endings. Management, therefore, relies on a multidisciplinary approach to chronic pain. Chronic pain services typically utilise a step-up pharmacological approach, starting with non-opioids (eg paracetamol, ibuprofen), progressing to mild opioids (eg codeine, tramadol) and, finally, strong opioids (eg morphine, oxycodone). Many of these patients become opioid-dependent. Imaging-guided coeliac plexus block is another option.14,15
Given that the underlying pathogenesis of chronic pancreatitis is one of sustained oxidative stress, some studies have suggested that a combination of antioxidants (eg vitamin C, vitamin E, selenium and methionine) may be a useful therapeutic adjunct to analgesia, although further, high-level evidence from randomised controlled trials is needed.16
Malabsorption
Occasionally, chronic pancreatitis may initially present as otherwise unexplained weight loss. Further questioning may reveal a history of chronic pain (as noted above) and/or steatorrhoea (loose, bulky, offensive stools). This is due to malabsorption engendered by loss of pancreatic exocrine parenchyma. Treatment consists of pancreatic enzyme replacement therapy (PERT), for which a number of commercially available digestive enzyme combinations are available. Although these are mostly enteric-coated, gastric acid can degrade their potency, and acid suppression therapy may be a useful adjunct.17 The dosage of PERT should be titrated according to symptom relief. Once malabsorption is corrected, there should be no need for additional dietary modifications.
Where symptoms are not clear-cut, pancreatic exocrine insufficiency (PEI) can be objectively diagnosed simply and effectively by measuring faecal elastase-1 concentration. A concentration of 200 µg/g is considered the lower threshold for moderate-to-severe PEI.9 That said, the role of PERT in alleviating chronic pancreatitis pain per se remains unclear.18 However, two prospective cohort studies have suggested that it reduces the extent of steatorrhoea and pain, and is associated with a significant improvement in quality of life.19,20
Diabetes
It is rare for the primary presentation of chronic pancreatitis to be recent-onset insulin-dependent diabetes. The islets of Langerhans are usually last to succumb in the progression of the chronic pancreatitis. One is nevertheless behoved to check blood glucose levels as part of the work-up of patients presenting with other symptom complexes. Levels should also be monitored as part of a chronic disease management protocol. When present, pancreatic diabetes may be no more difficult to manage than other types of diabetes, but referral to a specialist diabetes clinic is warranted for initial stabilisation and education.21,22
Acute pancreatitis
In a cohort of incident cases of acute pancreatitis in Far North Queensland, 54% of acute pancreatitis admissions were deemed to have underlying chronic pancreatitis on the basis of a composite of morphological, functional and clinical features.23 Indeed, some authors have proposed that even one episode of acute alcohol-related pancreatitis infers chronic pancreatitis.24 There is also a small but significant group of patients recovering from severe acute pancreatitis, from whatever aetiology, in whom there may be long-term deficits in exocrine or endocrine function.25 It is worth considering this when a patient returns to see their general practitioner (GP) following an admission for acute pancreatitis. If the precipitating aetiology was gallstones, best practice guidelines26 would dictate that a cholecystectomy should have been performed during index admission, or at least with minimal delay, thereby eliminating further attacks. The GP may serve as a safety net to ensure this occurs. All other cases should be reviewed from a chronic disease perspective. In other words, exocrine or endocrine function should be objectively assessed clinically and/or biochemically, with ongoing therapy and monitoring as indicated. Most importantly, potential risk factors should also be mitigated. In most cases, this means curbing, if not eliminating, alcohol intake. Unlike cirrhosis, pancreatitis has no known safe threshold for alcohol consumption. It is reasonable, therefore, to tell patients in whom alcohol intake was implicated, that their pancreas is ‘sensitive’, possibly because of genetic variations, which are becoming increasingly recognised and a possible focus for personalised medicine.27,28 In the interim, assistance from alcohol and drug rehabilitation services may be warranted.
Even in the absence of malabsorption, a certain dietary prise-en-charge may be indicated for patients with recurrent attacks of acute pancreatitis. In the Far North Queensland cohort, it was found that before the onset of symptoms heralding an acute attack, patients with underlying chronic pancreatitis were more likely to substitute food-based intake for combinations of non-nutritive substances such as tobacco and coffee, this being independent of alcohol intake.12 Although this may reflect a binge-type behaviour set, it may nevertheless be worth educating such patients on the need for maintaining a regular, balanced nutritional intake to prevent acute recurrences.
Common to all the above scenarios is the need for a psychosocial prise-en-charge, which may also have a cultural competence dimension. Many patients have a history of past or current alcohol dependence, and many long-term patients are opioid-dependent.
One of the greatest obstacles to chronic disease management in the case of pancreatitis is the difficulty in achieving compliance with follow-up. It may be that any management should be done in an opportunistic manner, seeking ‘teachable moments’ in the course of a patient’s disease. Further, the concept of prise-en-charge should also include empowering and educating patients to take charge of themselves, in collaboration with the clinician.
A final word about pancreatic cancer
Chronic pancreatitis, regardless of aetiology, has long been identified as an independent risk factor for pancreatic cancer.29 There is no cost-effective screening protocol for these patients. However, in the course of follow-up, new-onset diabetes or a change in the nature or intensity of chronic pain should lead to re-imaging.
Case: The chronic implications of severe acute pancreatitis
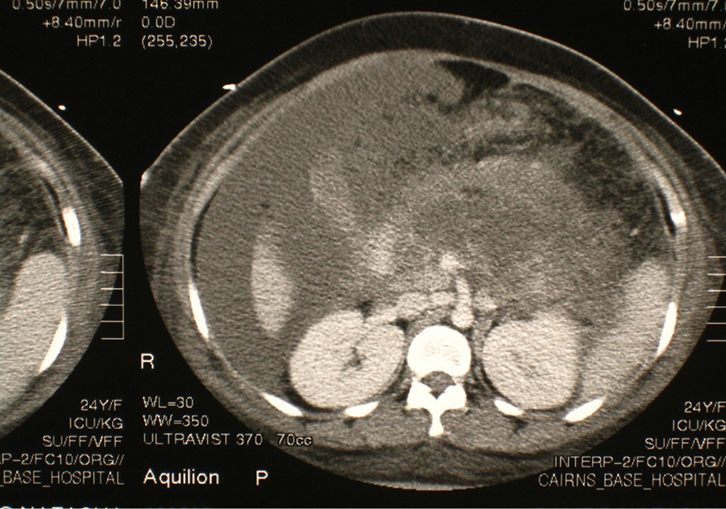 |
| Figure 1. CT image showing extensive pancreatic necrosis and intraperitoneal fluid collections |
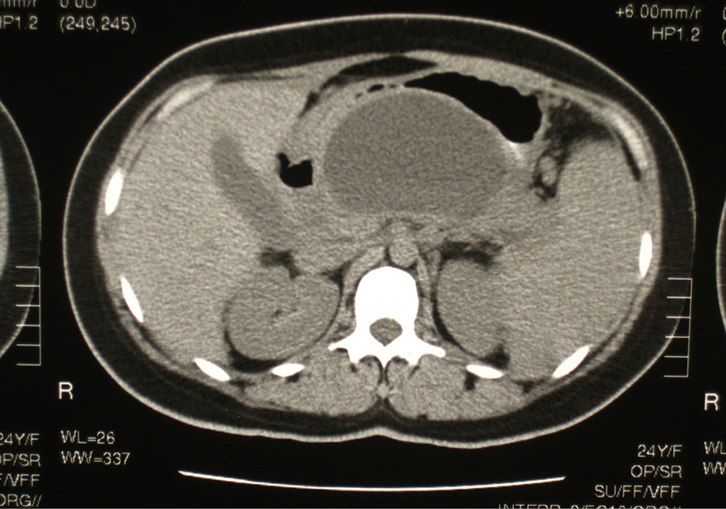 |
Figure 2. CT image showing large pancreatic pseudocyst occupying the
lesser sac |
|
A woman of Torres Strait Islander origin, 24 years of age, was admitted to hospital with a 2-day history of worsening upper abdominal pain radiating to the back, with associated nausea and vomiting. There was no significant past medical or surgical history. She was a non-smoker and drank alcohol only very occasionally. On admission, she was afebrile. Other vital signs were within normal limits. Abdominal palpation revealed tenderness and guarding in the epigastrium. A diagnosis of acute pancreatitis was confirmed by a serum lipase level of 13,600 IU/L (8–78 IU/L). The total serum bilirubin was 60 µmol/L (<25 µmol/L), and the serum levels of all liver enzymes were slightly elevated. In order to establish the aetiology of the attack, an ultrasound scan of the upper abdomen was performed. This showed multiple small gallstones in the gallbladder, but the biliary tree was not dilated.
The patient was treated with nil-by-mouth, intravenous fluids and analgesia. Oral intake was re-introduced as the pain settled, and the episode of acute biliary pancreatitis resolved after 3 days, at which time she was discharged. To prevent further attacks of acute pancreatitis, it was planned to re-admit her for a laparoscopic cholecystectomy in the near future. However, this procedure was postponed because there were more urgent cases on the elective operation waiting list.
On the eve of her operation, 3 months after the initial presentation, the patient developed further abdominal pain. Serum lipase was measured at 8000 IU/L (8–78 IU/L), thus diagnosing acute pancreatitis again. On this occasion, despite initial conservative management, she progressed to develop severe acute pancreatitis, as evidenced by respiratory failure and extensive peripheral oedema (due to falling serum albumin levels). This required admission to the intensive care unit, for endotracheal intubation and positive pressure ventilation. A CT scan of the abdomen with intravenous contrast was taken to ascertain the state of the pancreas. This showed extensive necrosis (Figure 1). Given the possible complication of infected necrosis, CT-guided fine needle aspiration of the non-enhancing pancreas was performed for microscopy and culture. However, this proved to be sterile. Additional management included nasojejunal alimentation. Haemodialysis was not required. The total time of hospital admission on this occasion was 7 weeks.
Six weeks following her discharge from hospital, the patient was seen as an outpatient, having had a follow-up abdominal CT scan. This revealed a moderate-sized pancreatic pseudocyst in the lesser sac of the peritoneal cavity (Figure 2). At this time, the patient was complaining of a mild, upper abdominal bloating sensation and early satiety. On abdominal palpation, there was minimal epigastric tenderness and the impression of a firm, non-pulsatile mass. An open cholecystectomy and cystogastrostomy were subsequently performed. The postoperative course was unremarkable.
Three months later, the patient stated that she was feeling well. Periodic random blood glucose levels were within normal limits. A recently measured faecal elastase-1 concentration was 210 µg/g. Although this borders on the definition of exocrine insufficiency (<200 µg/g), she reported no symptoms of malabsorption.
Case discussion
This case illustrates a typical course of severe acute pancreatitis with long-term sequelae. At least two salient points relevant to clinical management emerge. First, delaying cholecystectomy following acute biliary pancreatitis risks further attacks with more serious consequences. Various international consensus-based guidelines30–32 have recommended that after mild gallstone-associated acute pancreatitis, cholecystectomy should be performed as soon as the patient has recovered, preferably within the same hospital admission.
Second, a severe attack of acute pancreatitis has ongoing consequences that merit follow-up, as would be indicated for a chronic disease process. In this case, at least one return to a specialist clinic following discharge would be expected, given that pseudocyst is a well-recognised complication of necrotising pancreatitis, warranting surgical treatment. However, less attention tends to be paid to longer term consequences of pancreatic function, in specialist and primary care settings. Although the patient would appear to have adequate endocrine and exocrine reserves, these are likely to be borderline at best, and may deteriorate more rapidly over time. Regular monitoring would therefore be advisable.
A retrospective cohort study from Sweden33 found that, of patients followed up after an attack of severe acute pancreatitis (n = 35), over half had developed frank diabetes or impaired glucose tolerance, and one-quarter had signs of severe exocrine dysfunction. In a smaller case-comparison study from the same institution, diabetes or impaired glucose tolerance was significantly more prevalent following a single episode of severe acute pancreatitis, compared with mild acute pancreatitis.34 However, there were no apparent differences in long-term exocrine function or overall quality of life. Such studies tend to be limited by case numbers available for follow-up in single centres, and by the logistics of the follow-up itself. Ongoing studies in multiple sites and pooling of comparable data are desirable in order to provide generalisable evidence on which management guidelines can be based.
Key points
- A diagnosis of chronic pancreatitis should be considered in patients presenting with chronic abdominal pain, malabsorption, otherwise unexplained diabetes or acute pancreatitis.
- Diagnostic work-up should commence with a systematic history, incorporating range and severity of symptoms, and risk factors; this should be complemented by appropriate imaging.
- Functional tests may be indicated, based on symptoms.
- Management should be tailored to symptom relief and preventing disease progression.
- Management should be based on a chronic disease paradigm, ideally coordinated by primary care providers.
Author
Richard Turner MBBS, BMedSc, FRACS, PhD, Professor of Surgery, University of Tasmania, Hobart Clinical School, Hobart, TAS. Richard.Turner@utas.edu.au
Competing interests: None.
Provenance and peer review: Commissioned, externally peer reviewed.