Upper gastrointestinal (GI) disease is common in Australia. In Sydney, the prevalence of gastro-oesophageal reflux disease (GORD) symptoms is 12%.1 Worldwide, there is a rapidly increasing prevalence of GORD1 and Barrett’s oesophagus.2 Newly described pathologies are emerging, including eosinophilic oesophagitis (EoE).3 An Australian population survey has found that 7% reported wheat sensitivity without a formal diagnosis of coeliac disease.4 The diagnosis of upper GI disease and Barrett’s surveillance programs will often require endoscopy and biopsy. Accurate clinicopathological assessment is important for diagnosis and management of these diseases. In conveying a biopsy report to the referring doctor, the anatomical pathologist should ensure that the diagnosis and its significance are laid out in terms that are easily understood, and that lines of communication are open should there be confusion.
When to refer for endoscopy
The American Society for Gastrointestinal Endoscopy suggests four main indications for upper (and lower) GI endoscopies:5
- if a change in management is probable on the basis of endoscopy results
- after an empirical trial of therapy for a suspected benign digestive disorder has been unsuccessful
- as the initial method of evaluation as an alternative to radiographic studies
- when a primary therapeutic procedure is contemplated.
There are also screening or surveillance indications, for example, for Barrett’s oesophagus, oesophageal varices and biopsies to establish a diagnosis of coeliac disease. The presence of ‘alarm symptoms’ is another indication for endoscopy to exclude organic disease, although these symptoms are of limited value, as they have low sensitivity and predictive value for malignancy.6 Alarm symptoms or features include:6
- chronic GI bleeding
- progressive unintentional weight loss
- progressive difficulty in swallowing
- persistent vomiting
- iron deficiency anaemia
- epigastric mass
- older age group.
Why take GI biopsies at endoscopy?
Indications for GI biopsies taken at endoscopy include:
- detection of lesions (polyps, cancer, ulcers, Barrett’s oesophagus)
- obvious inflammation – EoE
- no obvious lesions, but to confirm/exclude coeliac disease
- suspected infection
- follow-up of cancer or polyps.
Endoscopists should ask the question of pathologists to which you want an answer – the history all important! The histology results should be ready two to three days after endoscopy.
The report and follow-up
The clinician who performed the procedure should take the necessary action required by the endoscopy report, but the general practitioner (GP) will also see the endoscopy and histology report, and should ensure that relevant treatment and follow-up occurs as necessary.
Interpreting the GI biopsy report
Oesophagus
Barrett’s oesophagus
Prolonged reflux can lead to Barrett’s oesophagus, a pre-malignant condition diagnosed by endoscopic and histological features. Barrett’s oesophagus is diagnosed when endoscopic features of columnar (glandular) mucosa and histological evidence of intestinal-type metaplasia (goblet cells) are detected.7 Dysplasia is particularly sought in biopsies and, ideally, confirmed by two independent pathologists.8 Although not without controversy, the recommended endoscopic surveillance schedule for the relevant endoscopic and histopathological findings in Barrett’s oesophagus is outlined in Table 1.8–10
It should be noted that adenocarcinoma (for which Barrett’s is a risk factor) is now more common than squamous cell carcinoma.
Table 1. Barrett’s surveillance
|
|
Endoscopic/histological finding
|
Surveillance interval/recommendation
|
|---|
|
Short segment Barrett’s (<3 cm),
no dysplasia
|
|
|
Long segment Barrett’s (≥3 cm),
no dysplasia
|
|
|
Barrett’s – low-grade dysplasia (LGD)
|
- 6 months
- If LGD persists, consider referral for endoscopic therapy; otherwise, repeat endoscopy every 6 months until clear of dysplasia
|
|
Barrett’s – high-grade dysplasia
|
- Immediate referral for endoscopic ablation9
|
|
Barrett’s – indefinite for dysplasia
|
|
|
Early adenocarcinoma, confined to mucosa
|
- Immediate referral for endoscopic treatment10
|
|
Invasive adenocarcinoma
|
|
Oesophagitis
Oesophagitis encompasses a wide range of inflammatory changes in the mucosa. Reflux oesophagitis is caused by GORD, and biopsy features include epithelial hyperplasia and inflammation, usually lymphocytes with few eosinophils (<7 eosinophils/high power field).
Eosinophilic oesophagitis (EoE)
Endoscopy may be normal for patients with EoE, but biopsy shows eosinophils, which are not normally present in squamous epithelium. More than 15 eosinophils per one high-power field are required for the diagnosis.11 EoE may be difficult to diagnose without a two-month trial of a proton pump inhibitor (PPI) and repeat endoscopy with biopsy.11 Topical, swallowed steroids (eg fluticasone) are the first-line pharmacological therapy for EoE and are well tolerated, although relapse is common.12 Oesophageal rings or strictures are potential complications of EoE and can be dilated at endoscopy (Case 1).11
Other causes of oesophagitis
Chemical, drug or pill-induced oesophagitis is the result of ingested poisons or oral medications, including nonsteroidal anti-inflammatory drugs (NSAIDs), antibiotics, quinidine, potassium chloride and bisphosphonates.13 Erosion or ulceration is seen during endoscopy, but the histological features can be non-specific and clinical history is most valuable in confirming the diagnosis (Table 2).14
Table 2. Drugs and the gastrointestinal tract
|
|
Drug
|
Biopsy findings
|
|---|
|
Nonsteroidal anti-inflammatory drugs, corticosteroids
|
Acute inflammation, reactive changes, non-specific erosions and ulceration13,14
|
|
Olmesartan
|
Lymphocytic gastritis, may mimic coeliac disease13
|
|
Iron tablets, anthranoid laxatives (including senna),
anti-hypertensive agents
|
Gastric erosions with iron pigment deposition13,30
|
|
Ipilimumab
|
Mimics coeliac disease14
|
Stomach
Dyspepsia and gastritis
Organic disease is an uncommon cause of dyspepsia symptoms as peptic ulcers are decreasing as Helicobacter pylori infection disappears.15 Only 10–15% of the Australian population are currently infected with H. pylori.15 Of note, however, is that if endoscopy is undertaken, biopsies for a rapid urease test and/or histology should be performed to look for H. pylori. Cultures can be taken for antibiotic sensitivity testing in treatment failure, as clarithromycin resistance is common and, when present, the success rate of regimens containing clarithromycin (including triple therapy) is only 10–30%.16
Other types of gastritis, such as reflux (chemical) gastritis, are due to duodenogastric bile reflux or NSAIDs.17 Chronic gastritis can persist following eradication of H. pylori infection, but is also seen in autoimmune gastritis, lymphocytic gastritis and gastritis associated with inflammatory bowel disease.18 Using current treatment guidelines,19 H. pylori should be eradicated for those who test positive. If a gastric ulcer is found on endoscopy, a repeat gastroscopy is recommended approximately eight weeks later to confirm healing and exclude gastric cancer, unless adequate numbers of biopsies were taken at the initial endoscopy (six to eight).20 There is no need to perform repeat endoscopy for duodenal ulcers.
Gastric polyps
Gastric polyps are uncommon and are usually asymptomatic and incidental findings at endoscopy.21 The most common are epithelial polyps, hyperplastic polyps, fundic gland polyps (FGPs) and adenomas. FGPs may be sporadic or associated with polyp syndromes (eg familial adenomatous polyposis [FAP]). Long-term studies have shown that the use of PPIs is not definitely associated with FGPs.21 Hyperplastic polyps are associated with chronic, chemical or reflux gastritis, H. pylori infection and pernicious anaemia.21 Suggested management and follow-up of gastric polyps is outlined in Table 3.22
Table 3. Gastric polyp surveillance
|
|
Gastric polyp type
|
Surveillance
|
|---|
|
Fundic gland polyp
|
- No dysplasia – no follow-up
- Dysplasia – consider familial adenomatosis polyposis (FAP) and perform colonoscopy
|
|
Hyperplastic
|
- Repeat gastroscopy in 1 year
- Test and treat for Helicobacter pylori
|
|
Adenomatous polyp
|
- Incomplete resection or high-grade dysplasia (HGD) – repeat gastroscopy in 6 months
- Complete resection and no HGD – repeat gastroscopy in 1 year
|
Gastric cancer
Gastric cancer is still the fourth most common cancer worldwide. Despite a steady fall in incidence and mortality rates in developed countries, it is a cancer that often remains asymptomatic until late in the disease, with associated poor outcomes.23 Gastric cancer is usually adenocarcinoma, with a heterogeneous pattern of growth, generally classified into three types – intestinal type, diffuse (signet ring type) or mixed – using Lauren’s classification.23 Surgery is the only treatment that can cure gastric cancer and is the treatment of choice for early-stage disease, with endoscopic resection for low-grade cancers and those confined to the mucosal layer.23
Duodenum
Duodenal biopsies are usually performed to exclude coeliac disease, as recommended by recent guidelines.24 Villous atrophy may be patchy – taking at least four biopsies doubles the diagnostic rate. A persistent positive serology with a negative biopsy should prompt re-biopsy, including the jejunum, and capsule endoscopy may be useful in this setting.24
Lymphocytic duodenosis
Lymphocytic duodenosis is defined by a normal villous architecture but an increase in intraepithelial lymphocytes (≥25 for every 100 epithelial cells). It can be associated with a number of conditions, including, but not limited to, coeliac disease, H. pylori infection and NSAID use. At the very least, this finding should prompt serological testing for coeliac disease.25 Mimics of coeliac pathologies are listed in Table 4.
Table 4. Histological mimics of coeliac disease: Either +/– villous atrophy and +/– increased intraepithelial lymphocytes (>25/100 enterocytes) to be considered in an appropriate clinical context29
|
|
Immune disorders
|
Immunoglobulin A (IgA) deficiency, hypogammaglobulinaemia
|
|
Autoimmune disease
|
Autoimmune enteropathy, Hashimoto’s thyroiditis, Grave’s disease, type I diabetes mellitus, rheumatoid arthritis, multiple sclerosis, systemic lupus erythematosus, haemolytic anaemia
|
|
Hypersensitivity/non-gluten protein intolerance
|
Protein intolerance (cow’s milk, soy, eggs, peanuts, cereals)
|
|
Infection
|
Helicobacter pylori gastritis, giardiasis, tropical sprue, post-infectious diarrhoea, virus, cryptosporidium, tuberculosis (TB) and atypical TB, acquired immunodeficiency syndrome (AIDS), Whipple’s disease
|
|
Drugs
|
Nonsteroidal anti-inflammatory drugs, proton pump inhibitors, chemotherapy, olmesartan
|
|
Neoplasia
|
Enteropathy-associated T-cell lymphoma, refractory coeliac disease
|
|
Other
|
Abetalipoproteinaemia, Crohn’s disease, eosinophilic gastroenteritis, glycogen storage disease, microscopic colitis (lymphocytic colitis, collagenous colitis), non-coeliac gluten/wheat sensitivity
|
Coeliac disease
Coeliac disease, with positive serology and confirmed on duodenal biopsy, is treated with a lifelong, gluten-free diet. Coeliac Australia has produced a useful guide for diagnosis, which shows the steps to be followed before referring a patient for biopsy.26 It is important that patients remain on a gluten-containing diet before biopsy, as withdrawal may obscure the pertinent histology findings of villous atrophy and increased intraepithelial lymphocytes. An algorithm for diagnosis of coeliac disease is shown in Figure 1.
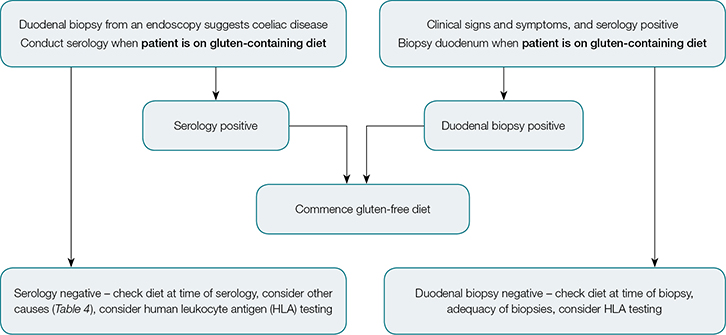 |
| Figure 1. Algorithm for diagnosis of coeliac disease |
Testing for a specific genotype can help to exclude coeliac disease as, for most patients, coeliac disease is associated with specific human leukocyte antigen (HLA) types (DQ2 and DQ8). Genotyping has a strong negative predictive value, as without the DQ2 and/or DQ8 genotype, patients are extremely unlikely to have coeliac disease.24 It should be noted, however, that 30–40% of Western populations express at least one susceptibility gene (which is thus not useful in confirming disease). HLA typing can be useful when biopsy and serology are inconclusive, if a patient is unwilling to have a gluten-containing diet for biopsy, or where there has been failure to improve on a gluten-free diet (Case 2).27
Food intolerance and allergy
Food intolerance, which affects 15–20% of the population worldwide, is defined as a non-immunological response to food components. Intolerance includes non-coeliac gluten sensitivity, enzyme defects and transport defects. Food allergy is defined as an immunological response to food components and may be mediated by immunoglobulin E or other mechanisms. Food intolerance states (eg lactose, fructose) and allergy (eg peanut and shellfish) are diagnosed by tests other than biopsy, and dietary exclusion trials are most useful for diagnosis.28,29
Duodenal infections
Duodenal infections can be important causes of chronic diarrhoea and malabsorption. Biopsy can sometimes show the infectious organism (eg giardiasis), but infectious causes often mimic coeliac disease (Table 4).
Drugs and the GI tract
Various medications, particularly NSAIDs, can cause histological damage to the GI tract. The effects of common medications in the upper GI tract are shown in Table 2.
Summary
Upper GI tract biopsies generate a wealth of different pathologies – subtle and florid inflammation, with nuances of dysplasia through to cancer – all of which require follow-up by the clinician. Therefore, accurate interpretation of the report is vital to the patient. If any doubt remains, communication between the clinician and pathologist is paramount.
Case 1
Mr EO, aged 27 years, complained of difficulty swallowing and a feeling of food sticking in his throat. He has had asthma since childhood. He presented to the emergency department with a food bolus obstruction. On gastroscopy, the oesophageal mucosa appeared to be ringed with longitudinal furrows (Figure 2A). Biopsies from the oesophagus showed oesophageal squamous mucosa with dilated intercellular spaces, mild basal hyperplasia, exocytosis of lymphocytes and >15 eosinophils/high power field (up to 65; Figure 2B), consistent with eosinophilic oesophagitis.
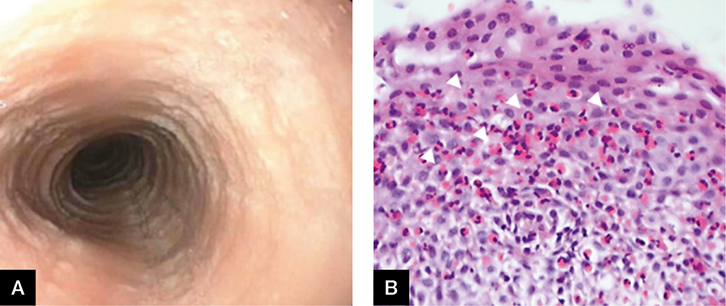 |
Figure 2. Gastroscopy
A. Ringed oesophagus; B. Histology of biopsy |
Case 2
Ms CD, aged 24 years, complained of bloating, abdominal pain and diarrhoea for the past two months. She lost 3 kg in weight and a blood test revealed iron deficiency anaemia. She has not altered her diet. On gastroscopy, the duodenum appeared atrophic, with loss of folds and scalloped mucosa (Figure 3A). Biopsies taken from the first and second part of the duodenum showed partial villous atrophy, increased intraepithelial lymphocytes (up to 60/100 enterocytes) and crypt hyperplasia (Figure 3B) consistent with coeliac disease. Correlation with tissue transglutaminase levels was recommended in the report.
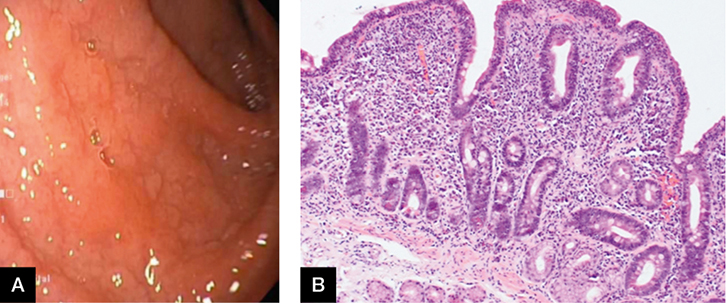 |
Figure 3. Gastroscopy
A. Atrophic duodenum; B. Histology showing partial villous atrophy |
Authors
Marjorie M Walker BMedSci, BMBS, FRCPath, FRCPA, AGAF, Professor, Anatomical Pathology, Faculty of Health and Medicine, School of Medicine & Public Health, University of Newcastle, Callaghan, NSW. marjorie.walker@newcastle.edu.au
Angela K Harris MBBS, PhD, Registrar, Pathology North Hunter, John Hunter Hospital, New Lambton Heights, NSW
Georgia C Edwards BSc, MBBS (Hons), Advanced Trainee, Department of Gastroenterology and Hepatology, John Hunter Hospital/Calvary Mater Hospital, New Lambton Heights, NSW
Nicholas J Talley MD, PhD, FRACP, FAFPHM, FRCP, FACP, FACG AGAF FAHMS, Pro Vice-Chancellor (Global Research), University of Newcastle, Callaghan, NSW
Competing interests: Marjorie Walker has received research support from Abbott, Janssen, Prometheus San Diego USA and Rome Foundation; consultancy support from Prometheus San Diego USA, Adelphi Values, Yuhan Corporation and Rome Foundation; and has consulted for GI Therapies and IM Health.
Provenance and peer review: Commissioned, externally peer reviewed.