FPOP is a common condition and has a lifetime risk for surgery of 10–20%.1,2 The aetiology is not fully understood;3 however, congenital factors play a role,4 and lifestyle factors such as obesity and smoking may contribute. Pregnancy and childbirth, especially vaginal delivery, are the most common modifiable risk factors,5,6 particularly for bladder and uterine prolapse, which are partly mediated through levator trauma.7 Use of forceps is the main modifiable obstetric risk factor.
Definition of FPOP
Pelvic organ prolapse is defined as downward displacement of pelvic organs, resulting in herniation of those organs into or through the vagina (uterovaginal prolapse) or anal canal (in the case of rectal intussusception and rectal prolapse). The former is divided into anterior compartment prolapse (usually a cystocele or bladder prolapse), uterine prolapse (which is termed ‘procidentia’ if complete) and posterior compartment prolapse, which may be a rectocele (a diverticulum of the rectal ampulla herniating into the vagina) and/or an enterocele (a herniation of the small bowel or sigmoid colon into the vagina). Prolapse is a hernia, and the hernial portal is the ‘levator hiatus’ (ie the opening in the pelvic floor muscle or ‘levator ani’, which allows the urethra, vagina and anorectum to transit the abdominal envelope).
Aetiology
The aetiology of FPOP was poorly defined until recently and there are still gaps in our knowledge. Vaginal childbirth probably plays a major role.6,8–10 Many pelvic reconstructive surgeons consider that prolapse is caused by distinctive fascial defects caused by vaginal childbirth.11 The concept is appealing because of its simplicity, and it provides a clear rationale for the reconstructive surgeon. Finding the defect, however, may not be so easy.
Another explanation is impairment of the levator ani through pudendal nerve trauma;12 however, there is little evidence of neuropathy in women with prolapse.13 Obesity is considered an established risk factor, but this may be true only for the posterior compartment.14 Similarly, ageing is thought to be a risk factor, although vaginal atrophy and urogenital involution are countervailing influences.15 Hence, it is not surprising that in some women prolapse is non-progressive.15 Conditions that lead to chronically increased intra-abdominal pressures, such as asthma and constipation, may also contribute.
There are variations in pelvic organ support within and between populations that are probably genetically determined.16–19 Genetic determinants of FPOP may be linked to collagen subtypes or connective tissue metabolism,20,21 but research has been inconclusive. At any rate, genetic risk factors are difficult, if not impossible, to modify. Additionally, no genetic prolapse study to date has controlled for obstetric trauma.
Vaginal childbirth is the main aetiological factor for FPOP. The largest potential hernial portal in the human body, the levator hiatus, is also the most critical soft tissue impediment to vaginal childbirth. The muscle forming this opening has to undergo a degree of distension that would rupture any other skeletal muscle,22 and it is surprising that major trauma occurs in only 10–20% of all primiparae after normal vaginal delivery or vacuum. This figure rises to 30–65% after forceps.23 In layman’s terms, the pelvic floor muscle is torn off its insertion on the pubic bone. The result is enlargement of the levator hiatus24 and an increased risk of FPOP,23 which may be difficult to treat.23
Symptoms
Many women with objective prolapse are asymptomatic and do not need treatment. Conversely, symptom bother may be considerable in some women.25 The most common symptoms associated with FPOP are those of a vaginal lump or bulge, or a ‘dragging’ sensation.26 In younger women, vaginal laxity is more commonly noticed and related to sexual dysfunction. Excessive movement of prolapsing tissues can cause dyspareunia. At times, a prolapse will impair voiding, which can occur with urethral kinking or be caused by urethral compression by a low cervix (especially if the uterus is retroverted), an enterocele or a rectocele.27
Posterior compartment prolapse may manifest with symptoms of obstructed defecation;28 rectocele (ie a diverticulum of the rectal ampulla) is the most common cause. If a rectocele is found in someone with bothersome obstructed defaecation, surgical treatment may be indicated even without symptoms of prolapse. Box 1 gives an overview of primary and secondary symptoms.
Box 1. Symptoms of prolapse
|
|
Primary:
Vaginal lump or bulge
Dragging sensation
Vaginal laxity or looseness
Dyspareunia
Secondary:
Straining to void, intermittent stream (due to urethral compression or kinking)
Straining at stool, incomplete bowel emptying and digitation (due to rectocele, enterocele or rectal intussusception)
Recurrent urinary tract infections (due to incomplete emptying resulting in a chronic residual volume)
Nocturia (due to accumulating residuals during the day)
|
Clinical diagnosis
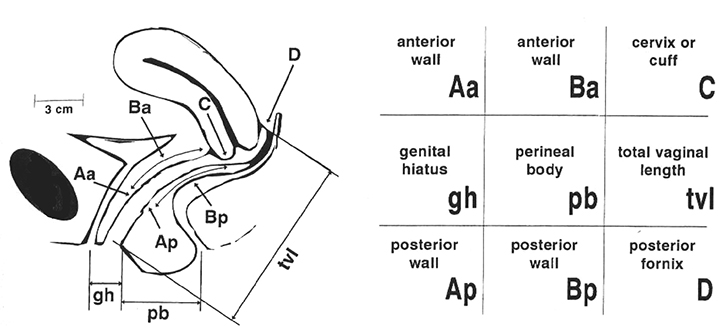 |
Figure 1. Prolapse assessment using the prolapse quantification system of the International Continence Society (POP-Q)
To simplify the task, many clinicians limit themselves to Ba for the leading edge of anterior prolapse, C for the leading edge of uterine or vault prolapse, Bp for the leading edge of posterior prolapse and tvl. The distance from external urethral meatus to anus (gh + pb) seems to be a good measure of ‘ballooning’ or hiatal distensibility58
Reproduced with permission from Elsevier from Bump RC, Mattiasson A, Bø K, et al. Am J Obstet Gynecol 1996;175:10–17 |
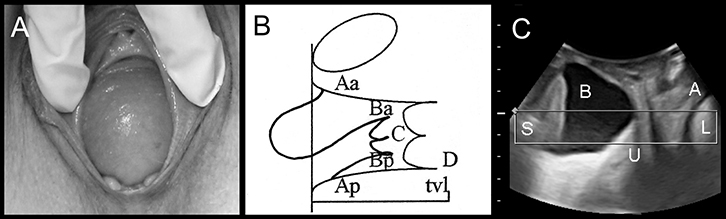 |
Figure 2. Anterior compartment prolapse
(A) Cystocele on clinical photograph
(B) Representation on POP-Q: Ba or leading edge of the anterior vaginal wall = +3, C = –4, Bp = –3)
(C) Appearances on imaging: S, symphysis pubis; B, bladder; U, uterus; A, anal canal, L= levator ani) |
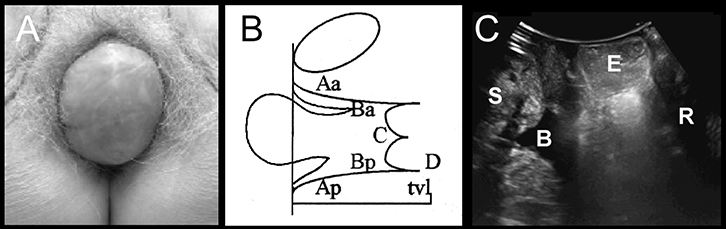 |
Figure 3. Central compartment prolapse
(A) Vault prolapse on clinical photograph
(B) Representation on POP-Q: Ba = –3, C = +2.5, Bp = –1)
(C) Appearances on imaging: S, symphysis pubis, B; bladder; E, enterocele; R, rectal ampulla |
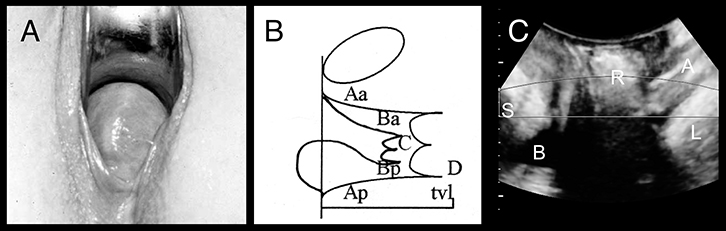 |
Figure 4. Posterior compartment prolapse
(A) Rectocele on clinical photograph
(B) Representation on POP-Q: Ba = –3; C = –4; Bp = +1
(C) Appearances on imaging: S, symphysis pubis; B, bladder; R, rectocele; A, anal canal; L, levator ani |
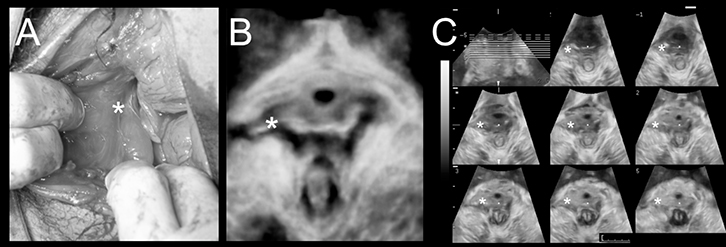 |
Figure 5. Levator trauma
(A) Delivery-related levator avulsion as seen on exploration of a large vaginal tear after vaginal delivery (*Defect)
(B) Delivery-related levator avulsion imaged on translabial 4D ultrasound 3 months later in a ‘rendered’ volume (*Defect)
(C) Tomographic imaging with 8 slices placed at 2.5 mm interslice interval
Adapted with permission from John Wiley & Sons Inc from Dietz HP, Gillespie A, Phadke P. Avulsion of the pubovisceral muscle associated with large vaginal tear after normal vaginal Delivery at term. A Case Report. Aust N Z J Obstet Gynaecol 2007;47:341–44 |
 |
|
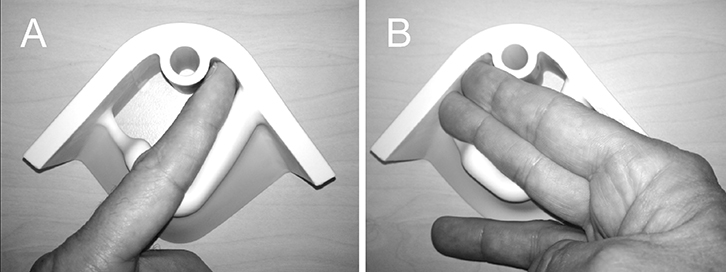
Figure 6. Model for teaching palpation of levator trauma
A finger is placed between the urethra and the pelvic floor muscle, palpating the inferior pubic ramus on which the puborectalis component of the levator ani inserts. Palpation is easier during active contraction of the muscle, which accentuates the muscle-bone interface. If the insertion is abnormal (ie if the muscle is detached from the pubic ramus) this results in a much wider space between the urethra and lateral sidewall (a wider ‘levator–urethra gap’ or LUG59), with no contractile tissue palpable on the inferior pubic ramus.
(A) Palpation of a normal LUG which admits one finger
(B) A full avulsion, with an LUG which is at least 2 fingers wide
|
FPOP is assessed on Valsalva, for the anterior vaginal wall in front, cervix or vault (after hysterectomy) in the middle, and posterior vaginal wall in the back. The most popular method is the Prolapse Quantification System (POP-Q) of the International Continence Society (Figure 1).29 It describes maximum descent of the mid-vagina anteriorly (point Ba; Figure 2), of cervix or vault (Point C; Figure 3) and of the posterior mid-vagina (Point Bp), relative to the hymen (Figure 4).
Measurements (in cm) below the hymen are positive and those above are negative. The system also measures vaginal length, genital hiatus (gh) from urethra to fourchette, and perineal body (pb) from fourchette to anus. The sum of Gh and Pb seems to be a measure of ‘hiatal ballooning’ (ie of the size of the hernial portal).30 Measurements are obtained by ruler or are estimated. Organ descent and ballooning should be assessed on maximal Valsalva, which should last at least 5–6 seconds.31 Fingers or a speculum can be used to reduce one compartment, allowing assessment of the others.
Findings should be reported as coordinates. Any values of Ba, C, Bp above –1, –5 and –1, respectively, can be considered normal. A Ba of more than –1 should be reported as ‘anterior vaginal wall descent to Ba = x.x’, and similarly for the posterior compartment. It is better not to use the word ‘cystocele’ or ‘rectocele’ as prolapse may be due to other conditions, which are discussed below. For the central compartment, reporting should be ‘uterine descent/vault descent to C = x.x. Gh + Pb can be reported as normal if it is <7 cm.30 Ballooning to 9 cm or more is common in women with major levator trauma32 but may be congenital or due to over-distension without actual muscle rupture.
Levator avulsion can be diagnosed by palpation during pelvic floor muscle contraction.33 Avulsion increases the levator–urethra gap, allowing it to admit not one but two fingers, and no contractile tissue is felt on the inferior pubic ramus. Imaging is usually required for a formal diagnosis, and tomographic three- or four-dimensional (3D/4D) pelvic floor ultrasonography is becoming the diagnostic standard (Figure 5). Figure 6 shows a model used to teach palpation of avulsion.
Diagnosis by imaging
This is performed by translabial ultrasonography, using abdominal curved array transducers placed in a mid-sagittal orientation on the perineum.34 The severity of FPOP is quantified against the symphyseal margin.35 Defaecation proctography has been the gold standard in the investigation of defaecatory symptoms but ultrasonography is better tolerated36 and cheaper, and can replace radiological techniques in the initial investigation of these women.37
Direct imaging of the levator is facilitated by 3D/4D ultrasonography, enabling diagnosis of avulsion and hiatal ballooning simply and non-invasively in an examination that takes, at most, 10 minutes and requires no preparation.23 As a result, it has become possible to define the likelihood of recurrence after conventional reconstructive surgery, allowing better counselling of patients and surgical planning.38 An ultrasound report should contain information about organ descent (eg cystocele to 2.7 cm below the symphysis pubis), levator integrity (eg right-sided complete levator avulsion) and distensibility (eg moderate ballooning to 33 cm2). The presence and status of implants should be specified (eg ‘there is a suburethral sling, probably a tension-free vaginal tape, in a typical position and not unduly obstructive’).
Primary prevention
Some aetiological factors for FPOP, such as obesity or genetic factors, are difficult or impossible to modify. Levator trauma, however, allows for two approaches, either avoiding vaginal childbirth through caesarean section or modifying it to reduce trauma. The first is not practicable except in individual cases, and attempts at selecting high-risk patients have been unsuccessful.39 The second approach seems feasible, but first attempts at preventing trauma via antenatal intervention have failed.40 Although several pathophysiological pathways remain to be explored, all will require substantial research efforts.
The use of forceps, the primary risk factor for levator avulsion, is entirely avoided in some countries and institutions, demonstrating that this risk factor is eminently ‘modifiable’. Odds ratios for levator avulsion in forceps relative to vacuum are 3.4–11.4,41 suggesting a large potential for prevention of pelvic floor trauma and FPOP, with the added benefit of less anal sphincter tears and anal incontinence.42
Until recently, it seemed that forceps delivery was becoming obsolete. In 1989, a review in the British Journal of Obstetrics and Gynaecology stated, ‘The obstetric vacuum extractor is the instrument of choice for operative vaginal delivery’.43 In Germany, a country with caesarean section and perinatal mortality figures similar to those in Australia, more than 90% of vaginal operative deliveries are done by vacuum.44 Forceps delivery is now similarly rare in the US, Sweden and Denmark, where rates have fallen to below 0.5%. There is some evidence that replacement of forceps by vacuum, as occurred in Denmark between 1960 and 1980, may substantially reduce the lifetime risk of prolapse surgery.45 However, this trend is being reversed in some jurisdictions. In England, forceps rates have doubled since 2004, from 3.3 to 6.8%.46 New South Wales seems to be following with a 5-year delay, and forceps rates in public hospitals have increased from 3.1% in 2008 to 4.3% in 2012.47 This is probably a consequence of an increasing bias against caesarean section.
Forceps provide a mechanical advantage – pull forces can be twice as high48 as those in vacuum, which means that some babies can be delivered by forceps that would otherwise require a caesarean section. In addition, there seems to be a trend towards increasingly difficult and rotational forceps deliveries in an attempt to reduce caesarean section rates. Kjelland’s rotational forceps seem to be particularly traumatic and the avulsion rate is more than 60%.49 Added to this is an increasing tolerance of long second stages and avoidance of epidural pain relief, both of which are likely to increase trauma rates.50 Episiotomy does not seem to be associated with increased trauma,51 but vaginal sidewall tears, and third- and fourth-degree perineal tears are markers for avulsion.52
Informed consent for performance of obstetric interventions needs to be considered. Given current evidence, it seems doubtful that many women would choose rotational forceps or even simple lift-out forceps over a vacuum, if presented with all the information. General practitioners (GPs) can play an important role by providing women with unbiased information, which may not be readily available or routinely discussed in the antenatal clinic setting.
Adverse events in childbirth are common. In a recent study only 25% of 443 low-risk primiparae with singleton births at term managed a normal vaginal delivery without major trauma.53 A common refrain of women seen in postnatal clinics is, ‘Why didn’t anybody tell me?’. This sense of disempowerment can be profound and contribute to postnatal depression and post-traumatic stress disorders in women after traumatic childbirth.54 Box 2 lists potential preventive measures.
Box 2. Primary prevention of prolapse
|
|
Pelvic floor muscle exercises (unclear status, no harm)
Perineal massage (unclear status, no harm)
Epi-No perineal trainer (no effect)40
Epidural analgesia (possible protection)50
Avoidance of forceps (risk reduction by about 20–40%)
Avoidance of vaginal delivery (risk reduction by 60–80%)
|
Secondary prevention
One could argue that anal sphincter or levator tears do not matter because we have no proof that intervention works. Such proof may take decades to obtain, given the long latency of FPOP.55 There are now data from a large intervention trial performed in women 12 years postpartum, showing that pelvic floor muscle training (PFMT) is effective in reducing prolapse symptoms and signs. As this trial included women with intact levator and normal pelvic organ support, the benefit is probably due to a larger effect in those women who actually needed the intervention (ie those with pelvic floor trauma).56
Treatment in primary care
Many women are not bothered by their prolapse, especially once its benign and often non-progressive nature is explained. If there is voiding dysfunction or obstructed defaecation, or if symptoms are bothersome, treatment is considered. In primary care, this involves lifestyle advice (weight loss, avoiding heavy lifting), bowel management advice and PFMT. The latter may increase bulk and/or resting tone of the levator, reducing symptoms,57 even in women who have not sought treatment.56 Hence, it makes sense to refer a patient with mild or moderate prolapse symptoms to a pelvic floor physiotherapist.
The next option is insertion of a vaginal pessary. There is a large variety of models but in primary practice, ring pessaries may be preferable, as they are unlikely to cause complications. As a rule of thumb it makes sense to start with a size just below or equivalent to Gh + Pb (ie the distance in cm between the urethral meatus and anus on Valsalva). We usually treat menopausal women with local oestrogen cream or ovula (per vaginam, twice weekly) and change the pessary every 3–4 months, at which time the vagina is inspected for erosion, which can give rise to discharge and spotting in menopausal women. In cases of erosion, we delay re-insertion by a fortnight to allow for healing. Self-management is sometimes possible.
Any treatment of prolapse may expose pre-existing weaknesses of the urinary continence mechanism. A poor urethra may remain continent if kinked by cystocele descent or compressed by a rectocele or enterocele. Prolapse reduction may cause incontinence, which may generate more bother than the original prolapse and also require surgery.
When to refer and to whom
Referral to a gynaecologist or urogynaecologist is indicated if:
- conservative treatment fails
- there are voiding problems or obstructed defaecation
- there is recurrent prolapse after reconstructive surgery
- there is ulceration or the prolapse is irreducible
- the patient prefers surgical treatment.
There is now a network of sub-specialist urogynaecologists in Australia who carry the Certificate in Urogynaecology (CU) qualification, and an increasing number of gynaecologists with a sub-specialty interest in this field.
Conclusions
FPOP is a common condition requiring surgery in 10–20% of women. Vaginal childbirth is the main aetiological factor, and major tears of the levator ani muscle (avulsion) seem to be the primary link between childbirth and prolapse of the bladder and uterus. Avulsion can be diagnosed by palpation, which,
together with prolapse quantification using the POP-Q system, is well within the scope of general practice. This is also true for conservative treatment with PFMT and pessaries.
Primary prevention is feasible through modification of obstetric management. The main modifiable risk factor for pelvic floor trauma and later pelvic organ prolapse is forceps, whereas vacuum is not associated with increased risk. Secondary prevention is feasible through pelvic floor physiotherapy, which requires provision of adequate diagnostic and therapeutic postnatal services. Such services do not currently exist. Until they are established, women with psychological or somatic morbidity after childbirth will benefit from a greater awareness of such morbidity and its causes among GPs.
Author
Hans Peter Dietz MD PhD, Professor of Obstetrics and Gynaecology, Sydney Medical School Nepean, University of Sydney, Nepean Hospital, Penrith, NSW. hpdietz@bigpond.com
Competing interests: Hans Peter Dietz has served as a consultant for Materna Inc and AMS in the past and has received grant support and had travel/accommodations expenses covered/reimbursed by GE Medical.
Provenance and peer review: Commissioned, externally peer reviewed.