Persistent pelvic pain (PPP) can be defined as pain in the area of the pelvis that has been present on most days for more than 6 months.1 No statistical data for the prevalence of PPP in Australia are available, but estimates of community prevalence in women range from 15% (USA)2 to 25.4% (New Zealand).3 With so many girls and women affected, general practitioners (GPs) will provide the majority of care for this condition.
The four components of PPP
PPP has similarities to and differences from other chronic pains. As for other types of chronic pains, there is:
- pain from end organs (pelvic organs)
- the musculoskeletal response to pain
- central sensitisation of nerve pathways
- the psychosocial sequelae of the pain condition.
In addition, PPP presents personal challenges above and beyond those experienced by others with chronic pain. The pain has embarrassing gender, fertility and sexual overtones, not easily discussed with family or friends. Patients look normal on good days, but stay at home, isolated and distressed, when the pain is severe.
Assessment of PPP
Pain from pelvic organs
One of the reasons that women find it difficult to get comprehensive care is that several organs may be contributing, and multiple comorbidities may be present. Care easily becomes fragmented across a range of healthcare professionals.
A common mix of comorbidities might include endometriosis (past or current), uterine pain (possibly with adenomyosis), painful bladder syndrome (interstitial cystitis), headaches including migraine and irritable bowel syndrome, possibly with recurrent candidiasis, food intolerance or vulvar vestibulitis.4–6
Symptoms suggestive of pelvic organ pain include:
- period pain
- urinary frequency, nocturia, urgency
- an irritable bowel
- recurrent candidiasis (where confirmed by vaginal swab and culture)
- vulval pain.
The musculoskeletal response to pain
Just as back pain is commonly complicated by muscle spasms, so is pelvic pain. However, the muscle groups affected may be intra-pelvic and their contribution to pain frequently undiagnosed.
Suggestive symptoms for pain from obturator internus include:
- stabbing pains felt on the sides of the lower abdomen that refer to the back or anterior thigh, which are worse with core strength exercise, make it difficult to walk when present, improve with heat packs and encourage a ‘fetal position’
- generalised pelvic ache
- pain aggravated by movement or prolonged positions.
Suggestive symptoms for pain from pelvic floor muscles include:
- stabbing pains in the vagina or lower bowel
- generalised pelvic ache
- difficulty initiating a void, or poor emptying despite urge
- pain with intercourse, speculums or tampons.
Central sensitisation of nerve pathways
Once pain of any kind is present on most days, central sensitisation of pain is likely. Brawn summarises the evidence for central changes in PPP.7
Suggestive symptoms include:
- pain present on most days, even if less severe
- bloated or burning feelings
- nausea, dizziness, anxiety, low mood, fatigue, poor sleep and unusual sweating
- ‘sensitivity’ of the lower abdomen where normal sensations such as tight clothes or touch become unpleasant or painful (allodynia)
- pain felt over a larger area when severe (wind-up pain).
Magnetic resonance imaging (MRI) has shown grey matter changes in a range of pain conditions including PPP.8
The psychological sequelae of persistent pain
A woman with PPP may have had pain for many years. Adolescence may have been accompanied by pain and self-doubt, and there may have been difficulties in personal development, relationships, sexual confidence, educational and financial opportunities. Such issues may have implications for medical management.
Suggestive symptoms of psychosocial difficulty include:
- withdrawal from social activities, study or employment
- hypervigilance to pain symptoms
- anxiety, depression, low self-confidence.
Examination of the patient with PPP
 |
 |
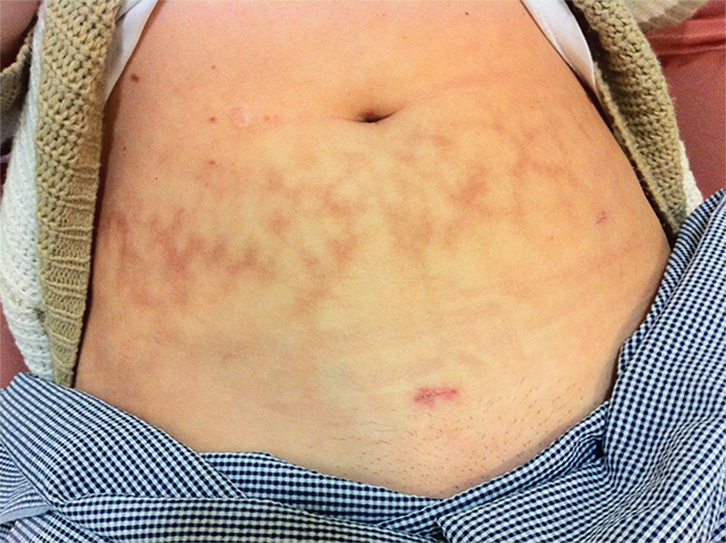
| Figure 1. Skin markings typical of extensive heat pack use |
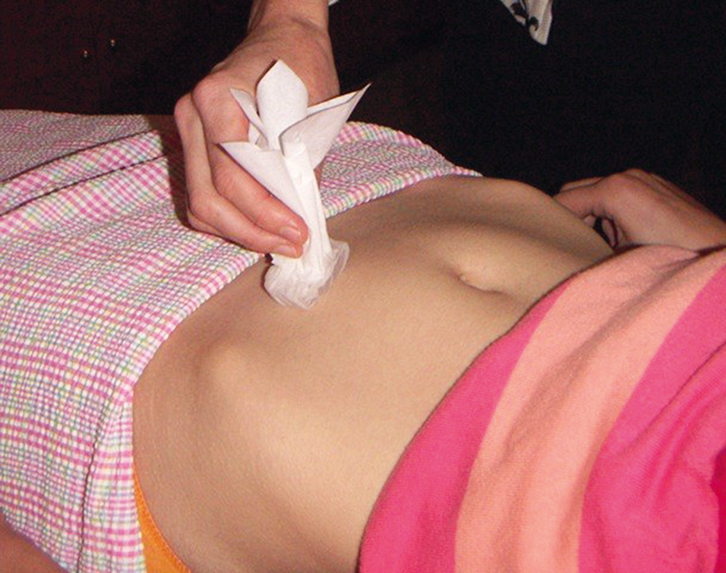
Figure 2. Mapping of cold sensation using wrapped moist ice cube |
The aims of examination are to assess the relative importance of each component of the patient’s pain and exclude infection. A sequence of examination may include:
- observation of gait: slow, awkward rising from a chair, a slow walk from the waiting room or sudden sharp pains are suggestive of pelvic muscle pain
- palpation of the lower back: gluteus medius, coccyx and sacroiliac joints posteriorly; tenderness is common in conjunction with intra-pelvic muscle dysfunction
- palpation of lower abdomen: for masses or tender points in rectus abdominus; Figure 1 shows the marks typical of extensive heat pack use
- assessment of cold sensation (optional): reduced cold sensation in the area of maximal pain may be present (Figure 2) and is suggestive of nerve pathway involvement
- visual assessment of vulval skin
- cotton tip swab assessment; vaginal introital sensitivity between 4 and 8 o’clock near Bartholins Glands suggests provoked vulvar vestibulodynia
- one-finger vaginal examination of the pelvic floor and obturator internus: the pelvic floor muscles are palpated (stroked) laterally just inside the vagina; the obturator internus is palpated slightly deeper at the level of the mid vagina by pressing laterally toward the hip; the right obturator internus will become tight and easier to palpate with your right forefinger when the patient’s flexed right knee is abducted laterally against your externally placed left hand; the left obturator internus is easier to palpate with your left forefinger vaginally and her left leg abducted laterally against your externally placed right hand. Where pelvic floor muscles are already tight, further contraction and then relaxation of the pelvic muscles around the examiners fingers on request may not be possible.
- vaginal examination: uterus and adnexae, then bladder and urethra through anterior vaginal wall
- speculum examination (if required): for exclusion of sexually transmitted infections
- vaginal swab: to exclude candidiasis or bacterial vaginosis; this may require, high vaginal, low vaginal and labial swabs.
Management of PPP
Once pain is persistent, a reduction in pain together with improved function and wellbeing may be more achievable goals than cure. Even so, substantial improvement is achievable. Jarrell et al9 describe the Canadian PPP management guidelines.
Using a questionnaire at the first visit helps determine which of the four components of pelvic pain are present. A suitable questionnaire can be downloaded from the Pelvic Pain Foundation of Australia (www.pelvicpain.org.au).
Managing pelvic organ pain
Dysmenorrhoea
If endometriosis, dysmenorrhoea or cyclical aggravations of pain are present, aim to minimise the number of periods, minimise the amount of bleeding and create a progestogenic (decidualised) environment. A monophasic oral contraceptive pill (OCP), oral progestogen (dienogest 2 mg or norethisterone 5 mg) or levonorgestrel intrauterine contraceptive device (IUCD) is preferred.10 The etonorgestrel implant may be effective if amenorrhoea can be achieved. For severe cases, dienogest 2 mg daily continuously has been shown to be non-inferior to GnRH agonists, has fewer hypoestrogenic side effects and improved quality of life.11
Amenorrhoea is optimal, but may require a combination of treatments (eg levonorgestrel IUCD and continuous OCP, or levonorgestrel IUCD and oral dienogest). Hysterectomy treats dysmenorrhoea well when fertility is no longer required but should not be considered as a cure for PPP. Dysmenorrhoea is often only one component of PPP.
Bladder symptoms
These symptoms may be due to a range of conditions; however, painful bladder syndrome is common.12 ‘Flares’ resemble urinary tract infections, but urine cultures are negative despite haematuria.
Management includes:
- assessing and excluding potential diet triggers (citrus fruits, fizzy drinks, caffeine, cranberries, artificial sweeteners, tomatoes)
- drinking 1.5–2 L fluid daily (mostly water) in normal weather
- acute management of flares (ie drinking 500 mL water mixed with 1 teaspoon bicarbonate of soda or two urine alkalinising sachets, then 250 mL water every 20 minutes for a few hours); antibiotics should be avoided unless infection is proven; providing a request form for urine culture if symptoms flare provides security that urine infection will not be missed
- use of medications including amitriptyline, oxybutinin, solifenacin and others, as outlined by Lau et al,13 amitriptyline has the added advantage of helping with sleep, headaches, the persistent pain condition, pelvic muscle pains and some irritable bowel symptoms, and is a good first choice.
Vulvovaginal irritation
Management depends on the conditions present and includes:
- avoiding soap/perfumed body wash – replace with QV/Cetaphil or Dermaveen body wash, and water only on vulval area
- exclusion of candidiasis – where repeated episodes of candidiasis have been proven, fluconazole 200 mg every 72 hours for
three doses then weekly for 6 months as a private prescription is effective14
- low-dose amitriptyline
- vulval dermatological review
- pelvic physiotherapy.
An irritable bowel
While bowel symptoms are common in women with endometriosis, it is uncommon for this to represent endometriosis of the bowel wall.15 Management includes:
- exclusion of red flag symptoms including blood or mucus per rectum, bowel incontinence, unexplained weight loss or malabsorption of food
- low FODMAP food diet16
- low-dose amitriptyline for bloated feelings.
Pudendal neuralgia
Pudendal neuralgia causes a burning or sharp pain in the ‘saddle’ area, anywhere from the clitoris back to the anal area, when sitting. It may be uni- or bilateral and may be associated with increased clitoral arousal.17 Management includes:
- avoiding activities that compress the nerve, such as cycling
- a ‘U-shaped’ foam cushion with the front and centre area cut out when sitting
- pelvic physiotherapy to downtrain pelvic muscles and reduce pressure on pudendal nerve
- ceasing straining with bowels or bladder
- neuropathic medications.
Managing pelvic muscle pain
Diagnosing the pain correctly may avoid unnecessary treatments and procedures. Management options include:
- avoiding aggravating activities (eg core strength exercise, prolonged positions)
- stretches (www.pelvicpain.org.au)
- pelvic physiotherapy to ‘down-train’ muscles
- optimising bladder and bowel function
- botulinum toxin injection for severe cases.18
Although becoming overtired is not helpful, giving up work rarely improves pain. It is best to keep active and keep moving. The physiotherapy adage that ‘motion is lotion’ remains true.
Managing central sensitisation
Management includes:
- an explanation that the nerve pathways have physically changed and become sensitised
- exercise – ‘the best non-drug treatment for pain’ (eg walking; where inactive, start with time outside each day, then a short daily walk with pacing to avoid over-tiredness)
- optimisation of sleep patterns19
- pain psychology
- neuropathic medications such as low dose amitriptyline, a serotonin-noradrenaline re-uptake inhibitor (SNRI) such as duloxetine, or an anticonvulsant such as pregabalin; in women, use small doses and increase slowly to a low peak dose (eg amitriptyline 5 mg 1–3 hours before bed, slowly increasing to 5–75 mg).20
Gillett and Jones20 provide a comprehensive and highly recommended discussion of medicines useful in women with pelvic pain. Initially, it is advisable to explain that each of these medications suits about half of those who take it, and that more than one type may need to be trialled, or a combination of two medications taken.
Managing the psychosocial sequelae of PPP
It is important to ensure the patient knows the GP believes in her pain and will take her concerns seriously.
Re-engaging with family, friends and community through activities with high motivational value for her will encourage mobilisation. This might include volunteering at a school if she enjoys being with children, study in an area that interests her, or craft activities she has always enjoyed. Exercise and meaningful activities help make pain a smaller part of her life.
A pain psychologist can consider life factors contributing to the overall pain experience. This includes a history of sexual assault, if present. Although the majority of women with pelvic pain have not been sexually assaulted, where this is present recovery is more complex.
Photographs from the patient’s laparoscopy can be used positively to illustrate the parts of her pelvis that are normal, or where ovaries are normal and fertility is unlikely to be severely affected.
Acute flares of PPP
Many women live with chronic pain every day. However, unexpected flares of uncontrolled pain, often causing the patient to fear that she must be in danger, result in presentations to the doctor or emergency department. Unfortunately, when the ultrasound, blood tests, pregnancy test and sometimes laparoscopy are normal, many leave without a diagnosis.
While ovulation pain, appendicitis and ectopic pregnancy (positive pregnancy test) are possible, common causes of acute flares of pain include:
- pelvic muscle spasm – often after an unusual activity/posture/exercise/pain or with severe period pain
- bladder pain – possibly after diet triggers or urinary tract infection on a background of urinary problems
- a functional ovarian cyst (usually only seen in patients not on OCP)
- a hormonally ‘different’ month – her period may be delayed
- recent severe stress with increased tension in pelvic muscles.
The role of opioid analgesics
Regular opioid use became popular with the rise of palliative care, where the patient’s condition was terminal. In patients with benign long-term pain, the development of dependence with regular use is common and use of opioids should be discouraged.
There is increasing evidence that opioids sensitise nerve pathways when used regularly.21 Darnell outlines the gender-specific risks and consequences of long-term opioid therapy in women.22 By contrast, nerve pathway (neuropathic) medications are more effective, can be used long term and may avoid the use of opioids.
The role of laparoscopy
Laparoscopy is an excellent tool for removing endometriosis, or for hysterectomy in older women with dysmenorrhoea. However, repeated laparoscopies increase the risk of exacerbating central sensitisation and surgical complications. Without specific indications for repeat laparoscopy, non-surgical options are preferred, at least in the first instance.
Where abnormalities are found at laparoscopy, these may or may not be the major cause of the current pain. Many causes of pain cannot be ‘seen’ at a laparoscopy and endometriosis is only one (albeit important) aspect of PPP.
Conclusions
Pelvic pain has been estimated to cost Australians more than $6 billion annually in direct costs.23 Early intervention has the potential to minimise its effects, but it currently remains unmentioned in Australia’s Women’s Health Policy.24 PPP truly represents a ‘hidden epidemic’. A GP with an interest in this area is in a good position to manage the majority of PPP symptoms.
Case
Chloe was a healthy and happy child until menarche, which occurred at the age of 12 years. Her periods were painful and heavy for 4 days every month. At 15 years of age, Chloe started taking an OCP, with improvement for 12 months before her periods became painful again.
After a particularly painful period, Chloe noticed the need to go to the toilet more often, and was waking up at night to void. Her periods were now painful for 7 days each month, her bowel was irritable and she frequently had nausea. She had poor sleep, and felt anxious, depressed and fatigued.
Stabbing pains began and she ceased playing netball or going out with her friends. Her mother, overwhelmed by concern for Chloe, stopped work, unable to fulfil the needs of her job.
By the age of 17 years, Chloe had pain every day, was missing most days of school, using regular paracetamol plus codeine, and spent her time in pain on the couch at home, isolated from friends.
Chloe has PPP. Although her pain may have begun as period pain, probably with endometriosis, she also has bladder and bowel comorbidities, pelvic muscle pain and central sensitisation. The condition has had a negative impact on Chloe and her family. Pain management and family rehabilitation in this situation can be achieved by managing all four components of her pain. Detailed patient information, medical instructions, pelvic muscle stretches and ‘tips for parents of teens with pain’ are available from the not-for-profit Pelvic Pain Foundation of Australia (www.pelvicpain.org.au).
A plan for Chloe’s pain
Pelvic organ pain:
- Reduce menses with an OCP or, if ineffective, either dienogest 2 mg or norethisterone 5 mg continuously, starting at day 5. Consider levonorgestrel IUCD (with continuous OCP if required) where available. Regular non-steroidal anti-inflammatory drugs (consider suppositories) for the worst 2–3 days of period may be used.
- Where period pain is severe despite OCP use, refer for consideration of laparoscopy to exclude/remove endometriosis.
- For an overactive bladder, low-dose amitriptyline, starting at 5 mg in the early evening should be considered. Ensure a mid-stream specimen of urine (MSSU) is sent for analysis if a urinary tract infection is suspected.
- A trial of a low FODMAP diet for her irritable bowel and intolerance of wheat/dairy foods could be considered after coeliac blood screen.
Pelvic muscle pain:
- Regular gentle exercise, starting with short walks daily. Encourage regular exercise that is ‘away from the core’ with a wide variety of gentle movements.
- Pelvic muscle stretches (illustrated at www.pelvicpain.
org.au).
- Pelvic physiotherapy to ‘down-train’ pelvic muscles, if available. This should not be painful.
Central sensitisation of nerve pathways:
- Do regular exercise.
- Consider medications including amitriptyline, an SNRI (eg duloxetine) or an anticonvulsant (eg pregabalin/gabapentin). Start with a very low dose, increase slowly to low peak dose to improve tolerance. If sedated, consider duloxetine.
- Refer to a pain psychologist.
- Avoid or reduce regular use of opioid analgesics, which sensitise nerve pathways.
- Improve sleep patterns.
Psychosocial sequelae:
- Encourage involvement in activities with high motivational value that the patient enjoys.
- Support parents in maintaining as normal an environment as possible (refer to ‘Tips for parents of teens with pain’ information sheet at www.pelvicpain.org.au).
- Manage anxiety/depression.
- Encourage school attendance and optimism.
- Recognise progress in symptoms and successes.
Be prepared to review symptoms when flares occur. Encourage participation in and education about her condition.
Author
Susan Evans FRANZCOG, FFPMANZCA, Gynecologist, laparoscopic surgeon and pain medicine physician at Pelvic Pain SA, Adelaide, and clinical lecturer, University of Adelaide, SA. drsusanevans@internode.on.net
Competing interests: Susan Evans has received monies for speaking and teaching engagements with Bayer and Pfizer, with proceeds donated to the Pelvic Pain Foundation of Australia. Susan Evans is a voluntary, unpaid director of the Pelvic Pain Foundation of Australia and has authored a book on Endometriosis and Pelvic Pain.
Provenance and peer review: Commissioned, externally peer reviewed.