Imaging the thorax
Modalities available for imaging chest diseases include chest X-ray, computed tomography (CT) and nuclear medicine, including ventilation–perfusion lung scanning and positron emission tomography (PET). Magnetic resonance imaging (MRI) is a standard tool for assessment of congenital cardiac and vascular diseases but at this time is not in general use for imaging primary diseases of the lungs. In many simple disease processes, such as uncomplicated infection, imaging may not be required. This article discusses the two most common techniques used to evaluate diseases of the chest and briefly mentions some lesser used or emerging techniques. The reader is directed to the online version of the article for additional illustrative figures of salient points.
Chest X-ray
The chest X-ray remains the starting point in the imaging armamentarium. It typically includes posterior–anterior (PA) and lateral views, and gives an overview of the lungs and cardiovascular system. For PA imaging, which is preferred, patients are required to stand with their arms around a rectangular imaging system (film cassette or electronic imaging system). Anterior–posterior (AP) imaging is used when the patient is non-ambulatory but usually results in reduction of image quality, including magnification of heart size and poorer detail of lung structure. The AP technique can obscure pathology that is present and produce artefactual opacities. It is always useful to view the imaging findings in the context of the clinical findings, to avoid overdiagnosis or the wrong diagnosis. For example, an infiltrate in the lungs might be fluid (oedema), pus, blood or cells (in malignancy); correlation with the clinical situation will usually discriminate between these possibilities.
Indications for chest X-ray
Box 1 lists the common indications for requesting a chest X-ray. This does not normally alter clinical management of asymptomatic patients, or those with upper respiratory tract infections, minor chest trauma, uncomplicated asthma or exacerbation of chronic obstructive pulmonary disease (COPD), acute-on-chronic chest pain and hypertension.1 Diagnosis of rib fractures is based on clinical assessment; rib views are rarely helpful and many rib fractures are not visible. In patients with pneumonia, follow-up chest X-rays are not required if the patient is improving and there are no suspicious features. Follow-up at 6 weeks is recommended if the patient is older than 50 years, is a smoker or has chronic lung disease.1
Box 1. Indications for chest X-ray
|
- Infection: exclude pneumonia, positive Mantoux test
- Major trauma: exclude widened mediastinum, pneumothorax and haemothorax
- Acute chest pain: exclude pneumothorax, perforated viscus, aortic dissection
- Asthma/bronchiolitis: when diagnosis unclear and/or not responding to usual therapy
- Acute dyspnoea: exclude heart failure, pleural effusion
- Chronic dyspnoea: exclude heart failure, effusion and interstitial lung disease
- Haemoptysis
- Suspected mass, metastasis or lymphadenopathy
|
Interpretation of the chest X-ray
Reading a chest X-ray is simple if a systematic approach is used. A typical checklist is shown in Box 2. Usually, there will be a working diagnosis based on clinical suspicion. This will guide the practitioner to the most important structures (eg lungs and cardiac/diaphragmatic silhouette in pneumonia), but using a checklist will ensure that pertinent findings are not missed.
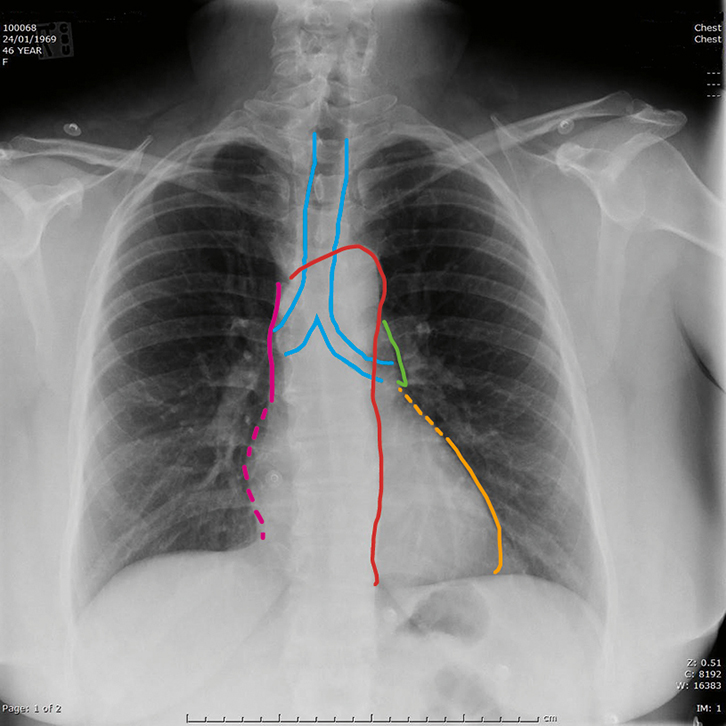 |
Figure 1. Normal posterior–anterior chest X-ray
The image shows the normal heart size (<50% of thoracic diameter) and uniform lung fields consisting of radio-dense and uniformly distributed vascular markings separated by the radiolucent gas filled airspaces.
Key to mediastinum: light blue outline, trachea and main bronchi; red, aorta; pink, superior vena cava; dotted pink, right atrium; green, pulmonary trunk; dotted orange, left atrial appendage; orange, left ventricle |
|
Box 2. Checklist approach to reading a chest X-ray
|
- Heart
- silhouette sign
- Mediastinum
- Diaphragm
- check recesses for effusion
- Lungs
- alveolar pattern: ‘dots’
- interstitial pattern: ‘lines’
- Skeleton
- check each rib
- check vertebral body height on lateral view
|
|
Figure 1 illustrates the components of the normal mediastinal silhouette. Becoming familiar with the normal structures that are seen forming the mediastinal contour will allow an appreciation of when the normal silhouette is lost, and point to a diagnosis. For example, in the case of consolidation, a part of the cardiomediastinal silhouette may become blurred by abnormally increased density of the fluid or pus-filled alveoli. Development of a mass in the mediastinum will result in alteration of the contour without loss of the sharply defined border. An understanding of the anatomical structures present in different parts of the mediastinum narrows the differential diagnosis of the origin of a mass. The mediastinum is divided anatomically into superior and inferior, based on the aortic arch. The superior mediastinum contains the airways, aortic arch and its branches as well as the superior vena cava and brachiocephalic vein. The mediastinum is also divided into anterior, middle and posterior compartments: anterior largely contains lymphatic structures; the middle mediastinum contains the heart and pericardium; and the oesophagus is the major occupant of the posterior compartment. Thus, a right superior mediastinal mass could arise from an abnormal vascular structure, lymph nodes, dilated oesophagus or enlarged thyroid. Where a lung mass abuts the mediastinum, it will obliterate the normal interface between air in the lung and the soft tissue density of the mediastinum; it may then appear to be arising from the mediastinum. Where a mass is suspected, CT is usually indicated.
Characterising lung infiltrates as either nodular (round) or reticular (lines) is the key to differential diagnosis of pulmonary infiltrates that, on initial assessment of patients and their chest X-rays, are not obviously caused by infective consolidation. Pulmonary oedema commonly presents as a linear infiltrate in the periphery of the lungs, usually in the lower zones (‘Kerley’ lines), caused by fluid accumulating in the interstitium of the lungs. Later in the course of decompensated cardiac failure, fluid will appear in the alveolus. This fluid may present as either a small nodular infiltrate 3–5 mm in diameter or as ‘fluffy’ confluent areas of consolidation when thousands of adjacent alveoli become filled with transudated fluid. Other causes of a small nodular infiltrate are atypical infection (including mycobacterial infection), sarcoidosis and disseminated metastatic disease. Correlation with the clinical scenario, therefore, is paramount. When a disease of the smaller airways or interstitium is suspected, the next imaging step is usually high-resolution CT.
Radiation dose
Table 1 shows the typical radiation dose from common imaging studies used to assess the thorax. The lifetime risk of inducing cancer from one chest X-ray is low, but we routinely adopt strategies to reduce the dose, such as omitting the lateral chest X-ray view in children and pregnant women. A ‘low-risk’ study (1 in 10,000 risk of fatal cancer) is one where the chance of death is only slightly greater than that resulting from road trauma in Australia.2 Further imaging in children is rarely needed as masses and interstitial diseases, for example, are relatively rare. General practitioners (GPs) should seek advice from a paediatrician or radiologist before ordering a CT scan in children, and be aware of possible alternative imaging modalities that use lower radiation doses (eg ventilation–perfusion lung (VQ) scanning for evaluation of young women for suspected pulmonary embolism).
Table 1. Radiation dose and associated risk of fatal cancer for common imaging studies of the thorax4
|
|
Test
|
Typical effective dose (mSv)
|
Equivalent natural background radiation
(1.5–2.0 mSv/year in Australia3)
|
Additional lifetime risk of fatal cancer*
|
|---|
|
Chest X-ray
|
0.1
|
18 days
|
Minimal
|
|
CT chest
|
7
|
3.5 years
|
Low
|
|
Bone scan
|
10
|
5 years
|
Low
|
|
PET/CT
|
25
|
12 years
|
Moderate
|
|
*Definitions of additional risk above the lifetime risk of fatal cancer in adults (1 in 5)
Minimal risk, 1 in 100,000 to 1 in 1 million
Very low, 1 in 10,000 to 1 in 100,000
Low, 1 in 1000 to 1 in 10,000
Moderate, 1 in 500 to 1 in 1000
|
Computed tomography (CT)
CT is usually the second step when the chest X-ray has identified an abnormality that requires further assessment, or when the disease course is prolonged and another condition is suspected. Box 3 lists common indications for ordering chest CT. Standard CT using current technologies actually uses the same minimum resolution as high-resolution CT, but is reconstructed with a larger slice thickness in multiple imaging planes. Thinner slices can be reconstructed to visualise smaller structures in finer detail. Thicker reconstructions are used to make small lesions (eg nodules) more conspicuous. Use of intravenous contrast is usually preferred but is contraindicated in patients with a history of anaphylactic reactions to iodine. Intravenous contrast should be used with caution in patients with underlying renal impairment because of the increased risk of contrast-induced nephropathy.
Box 3. Indications for CT
|
|
Evaluation of an abnormality detected on a chest X-ray
- Pulmonary mass or nodule
- Mediastinal mass
Evaluation of aortic disease
- Aortic aneurysm/dissection
- Trauma
Malignant disease
- Staging of primary tumour extent and its relationship to adjacent structures
- Detection of lymphadenopathy and metastatic disease
- Evaluation of metastatic disease where there is no known primary
- Assess suitability for biopsy
Evaluation of pleural disease
- Suspected pulmonary embolus
|
Patients should expect to be in the imaging department for approximately 30 minutes and will need to lie flat for about 10 minutes while being positioned and scanned. CT carries a higher risk of radiation-induced malignancy than a chest X-ray and should be considered only if it will change management.
Interpretation of CT scans
This is well within the capability of any primary care physician who is interested in mastering the skill. CT can be regarded as a high-resolution chest X-ray by separating into the mediastinum, lungs, pleura, skeleton and surrounding soft tissues.
Differential diagnosis is based on location and supplemented by morphological appearance and enhancement characteristics. Smaller lesions are much more easily appreciated on CT than on a chest X-ray. CT is superior to chest X-ray for assessment of pleural diseases. Figure 2 shows a chest X-ray and corresponding CT scan showing a loculated and benign pleural effusion.
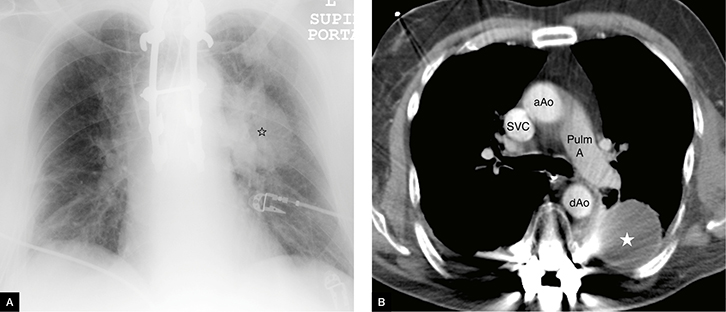 |
Figure 2. Loculated pleural effusion
A. Chest X-ray: loculated pleural effusions indicated by star; note the left pulmonary artery can be seen separate to the mass lesion
B. CT scan: pleural effusions indicated by star
aAo, ascending aorta; dAo, descending aorta; Pulm A, pulmonary artery; SVC, superior vena cava |
Indications for high-resolution CT
High-resolution CT is generally used for assessment of interstitial diseases. It is not generally used for assessment of solitary pulmonary masses, although it can be used to assess whether a small nodule contains calcification and, therefore, is most commonly benign. It is performed without intravenous contrast, using the same data acquisition technique but with a thinner reconstruction slice thickness, giving greater detail of the interstitium. Usually, the slices are separated by 5–10 mm such that masses arising between the slices may not be appreciated, so the technique is not suitable for assessment of malignant disease when used alone. Common indications for high-resolution CT are evaluation of suspected pulmonary fibrosis (where the chest X-ray is either normal or abnormal), solitary pulmonary nodule (PET/CT may be more accurate where nodules are larger than 8–10 mm), bronchiectasis and, finally, response to treatment of diffuse lung disease (eg alveolitis or bronchiectasis).
Bronchiectasis is an example of a disease process that is best assessed with high-resolution CT, although it is appreciable on standard CT. It is a disease that affects both small and large airways. The regional segmental and sub-segmental bronchi become dilated and thick-walled, and this is best appreciated by comparison with the unaffected lung. Smaller peripheral bronchioles appear either as tiny nodules scattered randomly in the sub-pleural lung or branching structures due to occlusion of the airway lumen, which is below the resolution of CT.
Other imaging investigations
The algorithm for assessment of a solitary pulmonary nodule or mass confirmed on CT now routinely includes positron emission tomography combined with CT (PET/CT). PET/CT is a powerful nuclear medicine molecular imaging technique for staging primary lung cancer (among other malignancies) where there is a moderate-to-high risk of malignancy.3 A number of different radioisotopes can be used, most commonly 18-fluorodeoxyglucose (18FDG). The intensity of uptake correlates with tissue metabolic activity. Most, but not all, lung cancers show increased uptake of varying intensity. However, uptake is not synonymous with malignancy; infection and sarcoidosis are other common causes of increased 18FDG uptake. GPs will now commonly read CT reports recommending PET/CT but this needs to be ordered by a specialist physician or surgeon.
Prior to surgery or commencement of neo-adjuvant therapies, a tissue diagnosis is usually required. CT can guide selection of the most appropriate biopsy method. For example, central lesions involving the airways are best approached using bronchoscopy or endobronchial ultrasound (EBUS)-guided biopsy, whereas peripheral lesions are often most successfully biopsied using CT guidance. Relative contraindications for CT-guided biopsy are bleeding diastheses or anti-platelet therapies, which may need to be withheld after discussion with the treating doctor(s).
CT pulmonary angiography (CTPA) is beyond the scope of this overview but is one of the techniques available for investigation of suspected pulmonary embolism along with VQ scanning.
Appropriate use of imaging
The options for imaging are becoming increasingly complex but there are several excellent online resources that GPs can freely consult in addition to their local radiologists. These include Australian resources such as the Diagnostic Imaging Pathways websites and North American websites, and offer comprehensive evidence-based information to guide selection of appropriate tests. They are usually based on evaluating a specific presenting complaint and can be complex to read. In the near future, it is planned that these resources will be integrated into electronic medical records to allow much easier use.
Key points
- Radiological investigation is not warranted in uncomplicated upper respiratory tract infection, asthma, minor trauma or acute-on-chronic chest pain.
- High-resolution CT is designed for assessment of lung detail and is poorly suited to assessment of the mediastinum or suspected malignancy.
- There are several comprehensive, evidence-based online tools to guide GPs in selecting appropriate imaging, but consulting a radiologist is often a simple first step.
Resources
Author
Sarah Skinner BMBS, FRANZCR, Clinical Director Medical Imaging, Bendigo Health Care Group, Medical Imaging, Bendigo, VIC. SSkinner@bendigohealth.org.au
Competing interests: None.
Provenance and peer review: Commissioned, externally peer reviewed.
 |
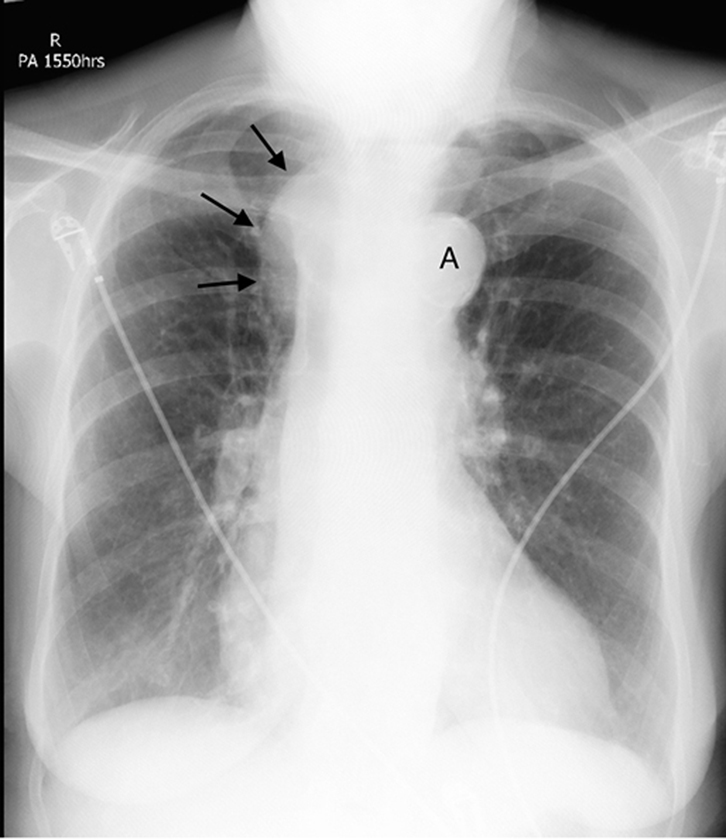 |
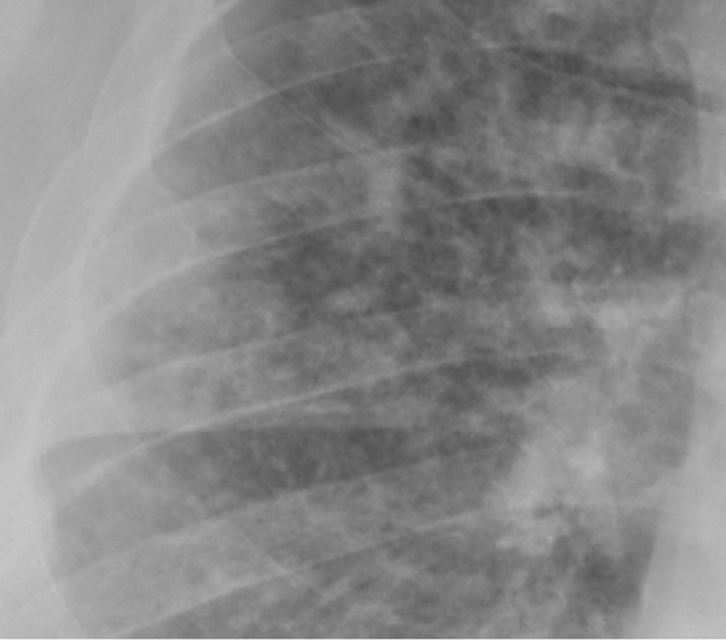
Figure 3. AP CXR in an elderly kyphotic patient with
acute pulmonary oedema shows a bilateral perihilar
or “bat-wing” infiltrate representing fluid leak into the
interstitium of the lungs |
Figure 4. CXR showing a right superior
mediastinal mass (black arrows) at the
level of the aortic arch (A) |
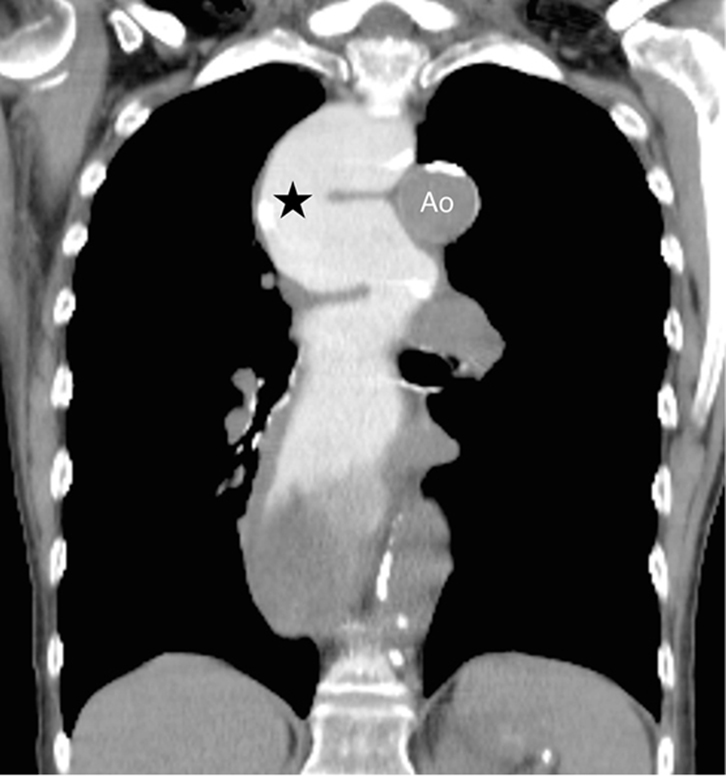 |
| Figure 5. CT chest with oral contrast of the same patient as figure 3 showing dense oral contrast in the dilated oesophagus (*). Note that the aorta (Ao) is not opacified as this CT has been performed without intravenous contrast. |
 |
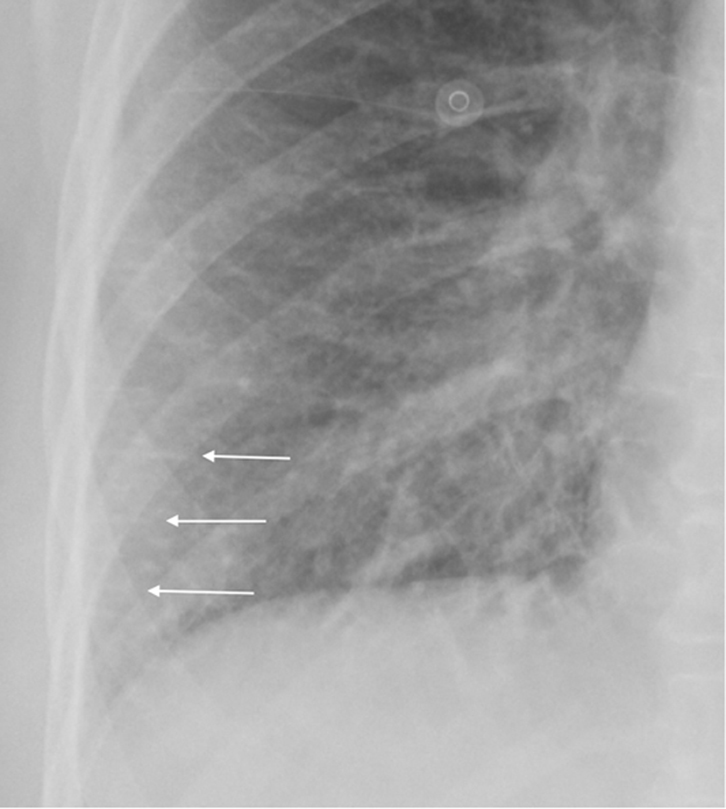 |
Figure 6. A magnified image of the right lung shows
small nodules of 3-5mm in size with ill-defined
margins in an immunosuppressed man with fungal
sepsis |
Figure 7. CXR (magnified) showing
“Kerley” lines due to abnormal fluid in the
peripheral interstitial space of the lung
(white arrows) |
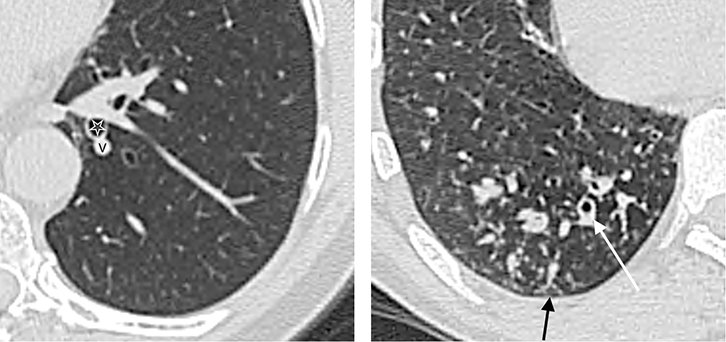 |
Figure 8. PET/CT shows an intensely-avid primary bronchogenic carcinoma
(A) and also identified an involved left hilar lymph node
(B) which was not enlarged on diagnostic CT. |