Sentinel node biopsy (SNB) has been widely accepted by surgical and medical oncologists to be the standard of care for patients with intermediate and thick melanomas. It can also be considered and discussed in patients with high-risk thin melanomas. This was reflected in an international consensus statement published in 2012.1 SNB has also been promoted in the National Health and Medical Research Council (NHMRC) guidelines since 2008, which recommend it be discussed with patients with intermediate and thick melanomas.2 The opinions expressed in a viewpoint article by Dixon et al in July 2014 diverged from this consensus statement and the common practice in oncology centres.1,3
The Multicenter Selective Lymphadenectomy Trial (MSLT-1) provides the highest level of evidence for evaluating SNB in melanoma patients.4 The trial involved 2001 patients who were randomised to have either SNB and completion lymphadenectomy if the SNB was positive, or observation only and delayed lymphadenectomy if there was lymph node relapse.
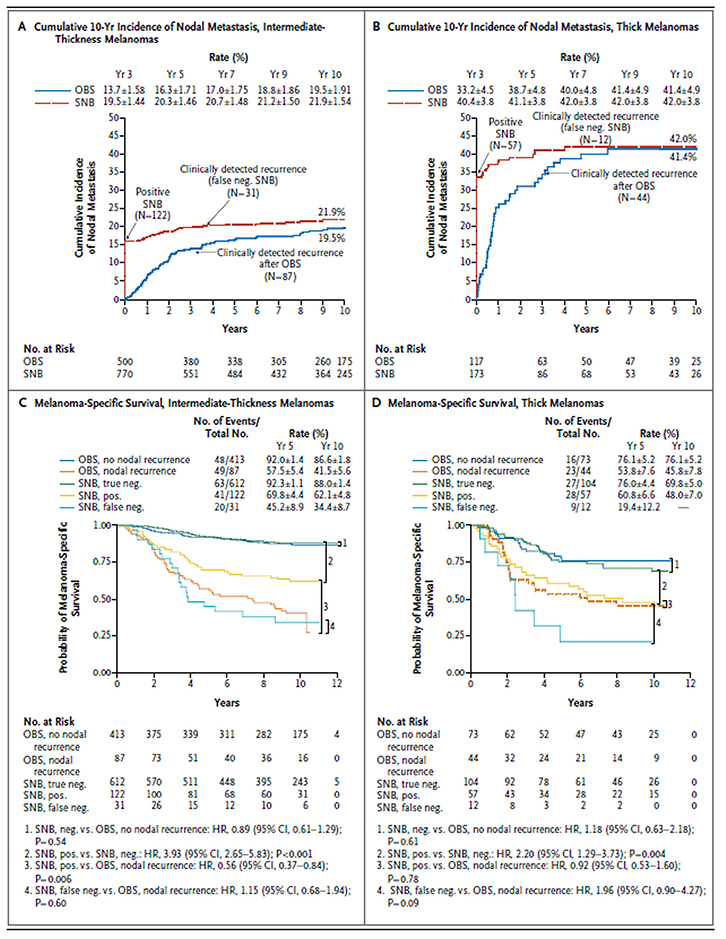 |
Figure 1. Estimated 10-year incidence of nodal metastasis and melanoma-specific survival, according to study group, melanoma thickness and presence or absence of nodal recurrence
Reproduced with permission from Morton DL, Thompson JF, Cochran AJ, et al. Final trial report of sentinel-node biopsy versus nodal observation in melanoma.
N Engl J Med 2014;370:599–609. |
Around 16% of patients with intermediate thickness melanoma (Breslow thickness 1.2–3.5 mm) were SNB-positive. However, 4% of patients had a falsely negative SNB result and tumours recurred later in the lymph nodes, despite the earlier negative SNB. Around 20% of patients in the observation group had lymph node relapses (Figure 1A).4 Around 41% of patients with thick melanomas (Breslow thickness >3.5 mm) in both groups had involved lymph nodes (Figure 1B).4 The similar frequencies of lymph node involvement is strong supporting evidence for the validity of comparing the two groups of lymph node-positive patients in intermediate and thick melanoma patients.
SNB is a diagnostic procedure that aims to identify involved lymph nodes earlier than clinical or radiological assessments. Clearly, removing a nodal metastasis earlier can only possibly improve survival in patients with lymph node metastasis present. There was no significant difference in melanoma-specific survival (MSS) between the two randomised groups in MSLT-1 (Figure 1C, D).4 Given that only 16% of patients who were SNB-positive could have had an impact on the survival of that group (if there were a benefit), and the event rate was lower than expected, the study was underpowered to detect a small difference (if it existed).
Although patients who have no lymph node involvement cannot benefit from SNB in terms of survival, they do benefit from more accurate prognostic information. In terms of MSS, the most important comparator groups in MSLT-1 were the SNB-positive patients and the (roughly) same proportion of patients who developed palpable lymph node metastases during observation. Clearly these groups could not be randomised, but the New England Journal of Medicine has accepted comparing their outcomes using previously validated statistical methodology.4,5
Since publication of the final MSLT‑1 results, most melanoma specialists in oncology centres continue to recommend that SNB remain the standard of care in patients with intermediate and thick melanomas and be considered in those with high-risk thin melanomas because:1
- MSS was improved in SNB-positive patients with intermediate-thickness melanomas who had an immediate completion lymphadenectomy when compared with those who subsequently developed clinically involved lymph nodes and underwent delayed lymphadenectomy (Figure 1C). MSS at 10 years improved from 41.5% to 62.1%.
- Sentinel node status is the strongest prognostic factor for MSS, as shown in multiple large studies, including MSLT‑1. It is now also known that factors relating to the degree of sentinel node involvement can further stratify 10-year MSS in a range from <40% to >90%.6
- SNB identifies patients with lymph node involvement early (Figure 1A, B),4 with the exception of false-negative cases. Patients who have a false-negative result (2–4% of all SNB cases)4 have a similar prognosis as those not undergoing SNB who later present with lymph node involvement (Figure 1C, D).4 Minimising false-negative SNBs is an important, ongoing process.
- The morbidity of SNB is low. Data collection for MSLT-1 was rigorous and most of the 10% morbidity reported were minor wound events.
- Completion lymphadenectomy in SNB‑positive patients has lower morbidity than delayed lymphadenectomy in patients with clinical lymph node recurrence. In addition, fewer lymph nodes, on average, are involved.4,7
- SNB is associated with improved disease-free survival (DFS) in patients with intermediate and thick melanomas. This means there is a longer time without disease relapse after the initial surgery.
- Potentially most importantly, SNB‑positive patients are candidates for adjuvant therapy. Melanoma management has entered a new era in which there is a range of systemic therapies with demonstrated efficacy in the metastatic setting. These are now being tested as adjuvant therapy. Selection of patients for adjuvant therapy trials requires knowledge of the lymph node status. An example of one such trial can be seen at http://clinicaltrials.gov/show/NCT01682083.
- MSLT-1 does not tell us whether SNB-positive patients need completion lymphadenectomy to achieve improved survival. This is being addressed in another trial, MSLT-2, which recently completed accrual. In the absence of high-level evidence, or enrolment in a clinical trial, completion lymphadenectomy in SNB‑positive patients remains the safest option if the patient is fit for the surgery. This is recommended in the Clinical Practice Guidelines for the Management of Melanoma in Australia and New Zealand2 and National Comprehensive Cancer Center guidelines (available at www.jnccn.org/content/11/4/395.full.pdf).
Patients with thick melanoma in MSLT‑1 had a 41% incidence of nodal disease. Of those in the observation group who relapsed, 93% relapsed first in the lymph nodes at a median time of 9.2 months.4 Distant metastatic disease tends to recur at a median time closer to 2 years. Hence, SNB in patients with thick melanomas improves DFS and quality of life, even if there is no impact on MSS. These patients also become candidates for adjuvant therapy trials.
In summary, there is convincing evidence for a survival benefit for SNB-positive patients with intermediate-thickness melanomas who undergo completion lymphadenectomy, compared with the similar percentage of patients who relapse in the lymph nodes on observation. Patients should also be given the opportunity to utilise SNB to gain prognostic information and improve DFS. For current adjuvant therapy trials, and when there is effective adjuvant therapy, SNB status will be required to select most of the patients who receive this treatment.
Authors
Andrew Spillane MD, FRACS, Associate Professor of Surgical Oncology, Northern Clinical School, Sydney Medical School, University of Sydney, Crows Nest, NSW; Surgical Oncology, Royal North Shore and Mater Hospitals, Sydney, NSW. andrew.spillane@melanoma.org.au
Rebecca Read MD, FRACS, Poche Fellow in Melanoma and Surgical Oncology, Melanoma Institute Australia, North Sydney, NSW
John Thompson MD, FRACS, Professor of Melanoma and Surgical Oncology, Melanoma Institute Australia, North Sydney, NSW
Competing interests: None.
Provenance and peer review: Not commissioned, externally peer reviewed.