Angiotensin-converting enzyme (ACE) inhibitors are widely prescribed in Australia, with mortality benefits in cardiovascular and renal disorders. Perindopril was the fourth most dispensed drug in Australia in 2010.1 ACE inhibitor use will potentially increase as it is a component of the ‘polypill’ currently in clinical trials as a preventive measure for people without cardiovascular disease.2,3
Extensive use of ACE inhibitors over many years has shown these drugs are generally well tolerated. Side effects range from a relatively trivial dry cough, dizziness, headache and/or nausea, to angioedema. Although uncommon, angioedema can be life threatening and present with airway obstruction.4
Other classes of medications associated with angioedema include non-steroidal anti-inflammatory drugs (NSAIDs), beta-lactam antibiotics, opiates and oestrogens. However, ACE inhibitors are the most common cause of drug-induced angioedema.5–7 The omapatrilat cardiovascular treatment vs enalapril (OCTAVE) trial found that angioedema occurred in 0.68% of patients on an ACE inhibitor for 6 months.8 Life-threatening airway obstruction occurred in 7–9% of these cases.9,10 The onset of the reaction can be immediate or delayed, occuring after months or years of uneventful treatment.11 Although angioedema has been recognised as an ACE-inhibitor-associated adverse effect since the mid-1990s, physicians may be unaware and not recognise the problem,12 occasionally with fatal outcomes.4,13 The absence of a clear, temporal relationship between the initiation of ACE inhibitors and the onset of angioedema may contribute to this. A lack of medical knowledge on ACE-inhibitor-associated angioedema has previously been identified in a knowledge assessment survey that included general practitioners (GPs).12
This article describes the clinical features, demographics and treatment of patients who presented to two South Australian hospitals with ACE-inhibitor-associated angioedema. It is hoped this will help to increase awareness of this uncommon but potentially serious side effect among GPs, who may only see a few cases in their individual practice.
Materials and methods
A retrospective case note audit was performed on patients who presented with angioedema to the emergency department at The Royal Adelaide Hospital and Flinders Medical Centre between 2008–10. Additional cases back to 2006 from Flinders Medical Centre were reviewed to achieve a total of 60 cases of ACE-inhibitor-associated angioedema. Each site had gained approval from their respective ethics committees (approval numbers 250.11 and 110415).
A detailed review of the case notes was performed with data extraction onto a pre-specified data collection form if the patient was prescribed an ACE inhibitor. The data collection form was adapted, with permission, from one used in a similar US study.10 Information collected included patient-specific factors (ie demographic, comorbidities, prior episodes of angioedema, other relevant medications), duration of treatment on an ACE inhibitor, and the time of onset of angioedema and symptoms. Secondary data collected included emergency department disposition, treatments used and any subsequent recommendations and/or course of action by the physician.
Results
Cause of angioedema
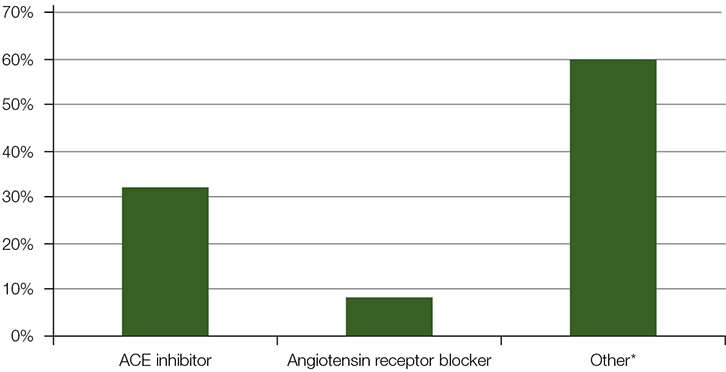
A total of 164 patients who presented with angioedema were identified across both sites between 2008–10. Fifty-one (31%; 95% CI = 24–39) patients were on an ACE inhibitor. Twenty-three additional cases of angioedema dating back to 2006 were reviewed at Flinders Medical Centre. This yielded an additional nine cases of ACE-inhibitor-associated angioedema, to make a total of 60 cases.
Demographics and medical history
The mean age of patients with ACE-inhibitor-associated angioedema was 72 years (range 42–93 years). There was an almost equal split between male and female cases (31 versus 29, respectively).
Most of the population were Caucasian (90%) and the rest were Asian (5%) or of unknown ethnicity (5%). Hypertension (92%) and chronic heart failure (8.3%) were the most common indications of ACE inhibitor use. Other comorbidities of interest were diabetes mellitus (13%), coronary heart disease (32%) and asthma (7%).
The duration of treatment with an ACE inhibitor prior to presentation varied from months to years. The onset of angioedema after the commencement of an ACE inhibitor was often delayed. One in three patients were initiated on ACE-inhibitor treatment more than 24 months prior to presentation, and only 15% for less than 1 month (Figure 2).
Almost one-third (27%) of patients had a history of at least one episode of angioedema prior to their presentation during the audit period. However, the cause of the previous episodes, or whether the patients were taking an ACE inhibitor at the time, are unknown. One patient had experienced four episodes of angioedema prior to their presentation during the audit period.
 |
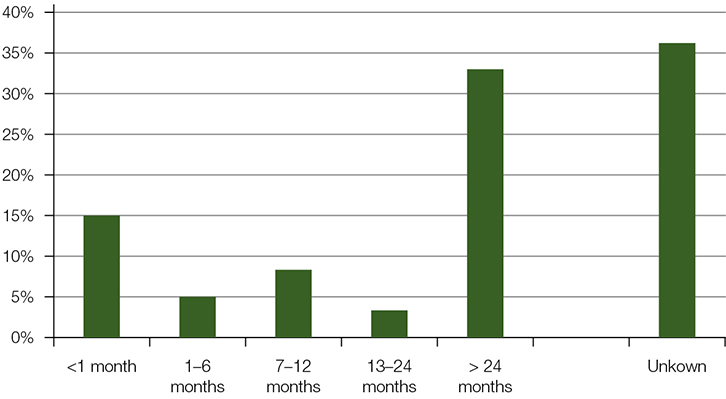 |
Figure 1. Suspected causes of angioedema in patients presenting to the Royal Adelaide Hospital emergency department (2008–10) and Flinders Medical Centre emergency department (2006–10)
*Other presumed causes (eg hereditary, idiopathic, other medications, food, allergens) |
Figure 2. Duration of treatment with ACE inhibitor prior to angioedema presentation |
Presentation and treatment
The time of onset of angioedema prior to presentation at the emergency department varied greatly (1 hour to longer than 12 hours), with no apparent predominant time of onset.
The prominent presenting symptoms were soft tissue swelling (98%) and respiratory distress (33%; Figure 3). Swelling commonly occurred on the tongue (73%) and lips (42%). Patients who presented with respiratory distress experienced voice change (21.7%), difficulty swallowing (20%) and shortness of breath (5%).
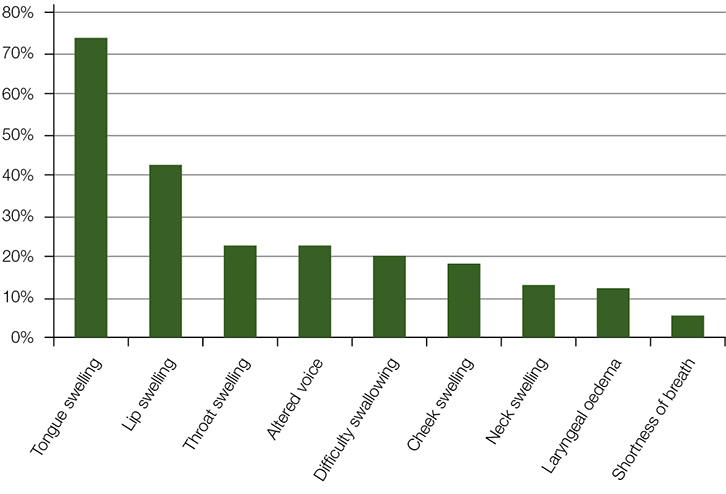 |
| Figure 3. Symptoms of ACE-inhibitor associated angioedema |
Infrequently, patients presented with urticaria (10%), which was generalised or on the hands, lips or throat.
Patients were most commonly treated with steroids (70%), antihistamines (65%) (of which 18 patients received intravenous promethazine), adrenaline (35%) and/or a H2-receptor antagonist (28%) (Table 1). Relatively few patients were treated with beta-agonists (10%).
In addition, patients were given supportive treatment, including oxygen (37%) and intravenous fluids (70%). Two patients required intubation in the intensive care unit because of severe respiratory distress.
Table 1. Treatments used
|
|
Adrenaline (IM, SC or inhaled)
|
35% (21)
|
|
Antihistamines
|
65% (39)
|
|
Promethazine
|
47% (28)
|
|
– IV
|
30% (18)
|
|
– IM or oral
|
23% (14)
|
|
Loratadine
|
17% (10)
|
|
Fexofenadine
|
7% (4)
|
|
Steroids
|
70% (42)
|
|
Prednisolone (IV, IM or oral)
|
20% (12)
|
|
Hydrocortisone (IV, IM or oral)
|
55% (33)
|
|
Dexamethasone (IV)
|
5% (3)
|
|
Oxygen
|
37% (22)
|
|
IVT
|
70% (42)
|
|
Intubation
|
3% (2)
|
|
H2-receptor antagonist
|
28% (17)
|
|
ß-agonist
|
12% (7)
|
Disposition and subsequent actions
More than half of the patients (55%) were discharged from the emergency department. Twelve patients were admitted to the intensive care unit, nine were sent to the high dependency unit for close observation and seven were admitted to the wards.
Following their presentation to the emergency department, 75% of patients had their ACE inhibitor discontinued and seven were referred to an allergist or ear, nose and throat specialist. One patient was re-admitted to the emergency department within 72 hours of discharge.
Discussion
Angioedema is a localised swelling of subcutaneous tissues, which may occur as part of an anaphylactic reaction or as a separate condition.4,14,15 This case note audit did not include patients with anaphylaxis as it was only concerned with patients who presented with angioedema in the absence of anaphylactic symptoms. There are several different types of angioedema, distinguished by their underlying pathophysiology and clinical presentation.14–16 This audit focused on patients presenting with angioedema in the context of being on an ACE inhibitor, which is typically a bradykinin-mediated phenomenon.14 Information on this uncommon but potentially life-threatening side effect is found predominantly in the emergency, intensive care or ear, nose and throat literature, but less in more general literature. The aim of this study was to increase awareness of the range of clinical features of ACE-inhibitor- associated angioedema in the primary care setting. GPs are frequent prescribers of this class of drugs.
Among the patients who presented to the emergency department with angioedema, 32% were on an ACE inhibitor. This is the largest case series of ACE-inhibitor-associated angioedema reported in Australia to date, with 60 cases reviewed.
The typical features of ACE-inhibitor-associated angioedema are subcutaneous swelling, usually around the head and neck, which evolves over several hours. It is not associated with itch or pain.4,9 Speech was altered in 22% of patients in this series, which may impair their ability to call for help. This is an important consideration for patients who are isolated or in remote areas of Australia. One in three patients experienced some form of respiratory distress in our case study, and two required intubation in intensive care. Previous studies have found no clear temporal relationship between the initiation of ACE inhibitor therapy and occurrence of angioedema.10,11
Our study was found to be consistent, with angioedema occurring months to years after the initiation of therapy in many patients. The onset of symptoms also occurred several hours prior to presentation to the emergency department. This can be clearly contrasted with anaphylaxis where symptoms usually develop more quickly.15
The pathological basis of ACE-inhibitor-associated angioedema is an elevation of bradykinin,17 a potent vasodilator that increases vascular permeability. Epidemiological studies have identified some risk factors predisposing patients to ACE-inhibitor-associated angioedema.9 People of African descent, a small population group in Australia, are up to four times more likely to develop ACE-inhibitor-associated angioedema.18,19 Other risk factors include being female, aged older than 65 years, smoking, NSAID (including aspirin) use and the presence of seasonal allergies.7,11 As it is not possible to predict which specific patients will go on to develop angioedema, clinicians need to remain vigilant for all patients taking an ACE inhibitor. Little is known regarding the risk of ACE-inhibitor-associated angioedema in Aboriginal and Torres Strait Islander peoples, although three severe cases, all with airway compromise, have recently been reported.20
There remains some uncertainty regarding the causal role of the ACE inhibitor in each individual case of angioedema. Association does not necessarily indicate causality. However, there is some evidence from epidemiological studies to assist with decision making for individual patients. The attributable risk factor for ACE inhibitor in patients with ACE-inhibitor-associated angioedema is 80% (95% CI = 51–92). There was a 10-fold increase in recurrence of angioedema in patients who had presented with ACE-inhibitor-associated angioedema and continued on ACE inhibitor, compared with those who stopped treatment.21 A recurrence rate of 50% was found in those patients who continued an ACE inhibitor for 5 years after an initial episode of ACE-inhibitor-associated angioedema. It also found resolution of symptoms in 85% when the ACE inhibitor was discontinued.22
Primary treatment of ACE-inhibitor-associated angioedema is discontinuation of the drug. Seventy-five percent of patients in this study were known to have their ACE inhibitor discontinued, although it is possible it was discontinued in more cases without documentation.
The common practice of treatments (eg corticosteroids, adrenaline and histamine blockers) may be ineffective as elevated levels of bradykinin mediate the presenting symptoms of angioedema.23 However, because angioedema is potentially life-threatening, and diagnosis of the cause may not be determined quickly, these drugs are often used as initial treatments.
Steroids and antihistamines were used in 70% and 65%, respectively, of our patients. Intravenous promethazine was used in 30% of patients, although this technique has been widely known to cause extravasation and thrombophlebitis and should be avoided. Relatively few patients in our series received adrenaline (35%). This may be a reflection of the older age of the study’s patients (mean age of 72 years) and concerns over cardiovascular risk with the use of adrenaline in this group. It may also reflect an understanding of the ineffectiveness of adrenaline for bradykinin-mediated angioedema, and the ability to observe the airway in the emergency department. In another large series of ACE-inhibitor-associated angioedema cases, only 9.5% of patients received adrenaline in the emergency department.10
The first priority is assessment and protection of the airway in the primary care setting. Education on the possible occurrence of angioedema is paramount for patients commencing ACE inhibitors. It is also important to advise patients to seek early review, particularly if there are concerns in relation to the airway. Early presentation of ACE-inhibitor-associated angioedema should minimise the possibility of severe airway obstruction, as it tends to develop slowly. Swelling is usually significant and clinically apparent in ACE-inhibitor-associated angioedema. Patients could be advised to take a picture of any swelling of concern for later review if they are unable to obtain medical review at the time of concern.
A recent approach is the use of icatibant, a bradykinin receptor blocker, in ACE-inhibitor-associated angioedema.23 However, its use is currently only approved for hereditary angioedema.
Our study has several limitations as it is a retrospective observational case series. We do not have any detailed information on the population from which the cases have come. For example, it is difficult to comment on the relatively equal sex distribution as it appears to contrast with other epidemiological studies that found an increased risk for females.7,11 We are unable to confirm the accuracy of the data on ACE inhibitor prescribing, as we do not have access to linked data. Therefore, it is possible we may have missed some cases of ACE-inhibitor-associated angioedema. The study does not have follow-up information to confirm if there had been resolution of symptoms with cessation of the ACE inhibitor. This would help with clarifying causality.
In summary, ACE-inhibitor-associated angioedema was responsible for 32% of angioedema presentations to two South Australian emergency departments between 2006–10. Presentations consisted mostly of painless soft tissue swelling, which developed over several hours around the head and neck, with occasional airway compromise. Onset was often delayed after commencement of ACE inhibitors. It is important for GPs to be aware of this adverse effect of a widely used class of medications.
Authors
Jimit Gandhi BPharm, Pharmacist, Royal Adelaide Hospital – Pharmacy Department, Adelaide, SA. Jimit.Gandhi@health.sa.gov.au
Rachel Jones MBBS, Medical Registrar, Flinders Medical Centre, Medicine, Bedford Park, SA
David Teubner BMBS, FACEM, M Clin Epi, Senior Staff Specialist, Flinders Medical Centre, Emergency, Bedford Park, SA
Genevieve Gabb MBBS (Hons), FRACP, Grad Dip Clin Epi, Royal Adelaide Hospital, General Medicine, Adelaide; and University of Adelaide, Medicine, SA
Competing interests: Genevieve Gabb has previously been paid for consultancy by the Therapeutic Goods Administration. She has also received payment from Astra Zeneca for lectures and had travel/accommodation/meeting expenses covered by the Heart Foundation.
Provenance and peer review: Not commissioned, externally peer reviewed.