The anatomical and functional aspects of multiparametric magnetic resonance imaging (mpMRI) enables its use as a biomarker that distinguishes between life-threatening and non-life-threatening cancer of the prostate. This imaging modality is a much more powerful risk-stratification tool than the prostate-specific antigen (PSA) test.
Long-term longitudinal studies (up to 15 years) are required to validate the impact of the image-based diagnostic pathway. Normal results need urological follow-up. Good collaboration between general practitioners (GPs), radiologists and urologists is essential.
PSA testing became available in Australia around 1990. Shortly afterwards,transrectal ultrasound-guided prostate biopsy (TRUSGB) was introduced. Over the subsequent 25 years, the investigative pathway for men with suspicion of prostate cancer – either with a high or concerning PSA, or an abnormal digital rectal exam (DRE) – involved taking multiple systematic but random biopsy samples from the prostate using ultrasonography as a guide.
In 1990, six biopsies was the standard practice. In the subsequent 25 years, the number of biopsy cores taken has increased to 12–32.1
This biopsy-driven pathway has identified more men with early prostate cancer and facilitated curative treatment at an early stage in the disease history. However, it has caused multiple problems that have been addressed in a series of taskforces and publications.2–6 The taskforces have recommended caution before patients have a PSA, as it may cause harm and might not increase their quality and length of life.
Despite this caution, PSA testing occurs freely in the community, as patients, GPs and urologists perceive a benefit, namely early diagnosis, and the hope of a cure.
The problems caused by the random biopsy-based diagnostic pathway are:7,8
- It misses some intermediate- and high-risk cancers.
- It finds (randomly) very small amounts of low-risk cancer that will never affect the patient’s longevity. This may lead to overtreatment and iatrogenic morbidity (eg urinary incontinence, erectile dysfunction).
- It sometimes finds low-risk cancer and misses high-risk cancer; the patient, therefore, is undergraded and may be undertreated.
- Millions of men around the world undergo biopsies because of raised PSA levels, most of which are negative, despite a significant risk of sepsis and recorded fatalities (the most common cause of a raised PSA is benign prostatic hyperplasia).
- Histopathologists have clearly documented their difficulty in interpreting the biopsy specimen in pathology.8
Recently, there has been a trend among urologists to change their biopsy technique from TRUSGB to the transperineal route (TPUSGB). The advantages of TPUSGB are that it reduces the risk of sepsis to minimal levels and is more accurate in detecting cancer. The disadvantages are that it continues to find low-risk disease, which leads to the chance of overtreatment. The problem is that the literature does not show a ‘disconnect’ between diagnosis and treatment. This means that many men around the world with low-risk disease are still being treated.9
The problem with telling a man he has low-risk cancer is that he only hears one word: cancer. This is a recipe for anxiety and leads to a high incidence of treatment. TPUSGB is not a minor procedure and requires a general anaesthetic. There are other complications such as post-biopsy urinary retention.10
Bringing prostate diagnostics into line with contemporary medical practice
The prostate is the only solid organ in the human body that has been subjected to such intensive interrogation with high-volume, random biopsies. The diagnostic pathway for other solid organs is based on imaging and a biopsy that targets the abnormal area shown on the image.
Prostate imaging has been suboptimal. Transrectal ultrasound (TRUS) and computed tomography (CT) show poor discrimination between cancer and benign pathology. MRI showed promise in its early days, but was expensive, difficult and time-consuming. It involved the use of a very uncomfortable endorectal coil and spectroscopy. Urologists regarded it as unsatisfactory.
In 2010–11, the European Research Radiologists began to improve their acquisition protocols and established quality-control mechanisms using a structured reporting system known as the Prostate Imaging-Reporting and Data System (PIRADS).11 They validated their reporting system by doing targeted biopsies in the MRI machine. This coincided with the introduction of three tesla (3T) magnets and improvements in software for processing images.
Contemporary MRI of the prostate uses three parameters (hence, the term ‘multiparametric MRI’ or mpMRI). The three parameters are:
- T2-weighted (T2W) sequences: this defines the anatomy and structure of the gland. The gland is examined with a three-dimensional cursor in the axial, sagittal and coronal planes. The peripheral zone (PZ) and the transitional zone (TZ) are examined individually.
- Diffusion-weighted imaging (DWI) sequences: under the magnetic field, water (hydrogen ions) diffuses through the prostate. Restriction of the diffusion speed indicates greater density in the tissue. Around 95% of life-threatening prostate cancers are denser than the surrounding prostate tissue.The software constructs:
- a ‘tissue density map’ called the apparent diffusion coefficient (ADC), where the tissue density can be quantitifed with an ADC number of 1–2000.
- a ‘high b’ value scan that reflects the distances the hydrogen ions travel in the denser areas of the prostate.
- Dynamic contrast enhancement (DCE): gadolinium is given as a bolus injection (contraindicated in patients with renal failure). The vascularity of the prostate is mapped. Multiple calculations are available, but the most common is ‘K-trans’, which reflects movement of the contrast from the vessels into the extracellular spaces of the prostate. This images new vessel formation of cancers and inflammatory changes in the gland.
The time taken to conduct a contemporary mpMRI is just under 25 minutes on a customised 3T magnet. Further developments will reduce the time. The aim is to make the examination less expensive.
The PIRADS score for mpMRI prostate
The overall PIRADS score is a weighted calculation on a 5-point scale. It is based on the probability that a combination of the mpMRI parameters (above) correlates with the presence of a clinically significant cancer at a particular location in the prostate.
PIRADS categories are as follows:
- PIRADS 1: very low (clinically significant disease is highly unlikely to be present)
- PIRADS 2: low (clinically significant disease is unlikely to be present)
- PIRADS 3: intermediate (presence of clinically significant disease is equivocal)
- PIRADS 4: high (clinically significant disease likely to be present; Figure 1)
- PIRADS 5: very high (clinically significant disease is highly likely to be present)
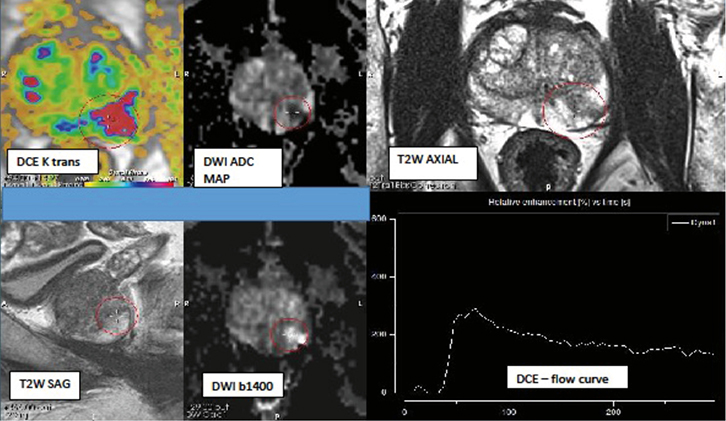 |
Figure 1. Illustration of an mpMRI, de-identified using PROCAD diagnostic platform
The parameters shown (T2W = 4/5; DWI = 4/5; DCE = +ve) best fit PIRADS 4 risk-stratifying to an 80–85% probability of being a clinically significant prostate cancer
The lesion is located in the left peripheral zone, mid-prostate |
The PIRADS scoring system was introduced by the European Society of Urogenital Radiologists (ESUR) after lesion appearance correlated with magnetic resonance (MR)-guided biopsies and radical prostatectomy specimens. It has now been endorsed by the American College of Radiologists (ACR). These organisations have recently published PIRADS version 2. The changes were made to increase the accuracy of the DWI by mandating high b value calculations and to help prevent overcalling of restricted lesions in the TZ of the prostate.
As in any complex pattern recognition system, there are recognised intra- and inter-observer errors in the interpretation of the images. There is also a significant learning curve of around 100 cases that need to be read independently and then co-reported by an experienced radiologist. A single reader has an accuracy of around 85%.12
There is an increasing role for mpMRI in the early detection of life-threatening prostate cancer, as well as monitoring patients with high PSA levels, active surveillance for patients with low- and intermediate-risk prostate cancer, and targeted biopsies of the prostate.
Targeted biopsies of the prostate
There are three recognised techniques to target lesions within the prostate.
MR-guided biopsy (MRGB)
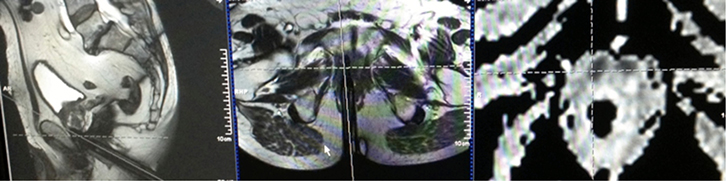 |
Figure 2. De-identified photograph of the console of the 3T Siemens Skyra
Customised biopsy software was used to target a small lesion anteriorly at the very apex of the prostate
The reference image (right panel) is an ADC map (part of the Diffusion study) showing a dense area anteriorly (black, in crosshairs); the deployed titanium needle is shown in the axial (orthogonal) plane (middle panel ) and sagittal plane (left panel); the needle is deployed just to the left of the crosshairs showing it is in the lesion. Histology revealed carcinoma with a Gleason score of 7 (4 + 3), which is intermediate to high-risk |
MRGB (Figure 2) is done in the MRI machine. It is the only technique using a direct ‘in-bore’ approach and has the greatest reproducibility. There are many advantages to this technique:
- Diffusion scanning (DWI and ADC map) can be used as a reference image, which allows the operator to target the most aggressive part of the prostate cancer. This will prevent undergrading and undertreatment, which can occur when a random biopsy reveals low-risk disease and misses the adjacent small, high-risk lesion.
- A validation image is made of the titanium needle within the target lesion. If the result is negative, the position can be assessed. If the needle is correctly placed, then the lesion is benign.
- It is a minor procedure and does not require anaesthetic or hospital admission.
- Once the operator is skilled in the techniques, biopsy of a single lesion takes approximately 20 minutes.
Disadvantages to this technique are:
- It is currently transrectal. However, in the lead author’s experience of 800 biopsies, the infection rate is very low (one case). This is because only two needles are generally used, and large benign prostates are not biopsied. The only two cases of sepsis after TRUSGB reported in the recent Australian trial of 223 patients12 were in PIRADS 2 patients with benign biopsies.12
- It requires access to a 3T magnet in the radiology department and experienced MR radiographers. There is a significant learning curve for the urologist or radiologist performing the biopsies. Availability is therefore restricted, but there is currently an upskilling of staff and an increasing number of magnets throughout the country.
TRUS/MRI fusion imaging techniques
The radiologist can merge the MRI and ultrasound images, which allows targeting under ultrasonography. This is an indirect technique and has a number of steps where small inaccuracies may be cumulative. Registration, segmentation and movement artefacts have hindered development, but the technology is improving. The advantage of this technique is that it allows the biopsy to be performed in the operating theatre or clinic. It still requires the purchase of an ultrasound machine with image fusion capabilities, a feature lacking in most ultrasound machines currently used for prostate biopsy. There is a learning curve for this technique, and cooperation between radiologists and urologists is essential. The same is true for MRGB.
MRI-ultrasound informed/cognitive
This technique involves the urologist looking at an MRI and judging where the lesion is located. They then target it to the best of their ability with ultrasonography, by using the shape and size of the prostate and anatomical landmarks to make a ‘best-guess mental picture’ of where the lesion lies. It is satisfactory for large but not for small lesions.
Multiparametric MRI acting as a biomarker
It became apparent that the mpMRI imaging was only identifying significant (life-threatening) cancer. Small volume and low-grade lesions were not being diagnosed. Initially, this was a criticism of mpMRI, but is now considered a major advantage for the patient. A patient with a normal mpMRI (PIRADS 1 or 2) may indeed harbour an insignificant or indolent prostate cancer. However, he will not know about it and will therefore not be treated for it. He will be observed with follow-up PSA and DRE over an appropriate time frame.
Multifocality of prostate cancer
Prostate cancer is often multifocal. It is generally accepted that the Gleason score determines the prognosis; the lesion with the highest score has the poorest prognosis. The ADC number (the lower the number, the more dense the tumour) reflects the Gleason score (the denser the lesion, the higher the Gleason score). The exceptions are mucoid-containing cancers and a rare Gleason 5 pattern without an increase in tissue density. These exceptions account for an estimated 5% of high-risk prostate cancers. Using DWI as a reference image, an MR-targeted biopsy of the most aggressive area of the tumour will, in most cases, reflect the highest Gleason score of the radical perineal prostatectomy specimen.
Current Australian research
Australian urologists began to take an interest in imaging in 2011 but were scared by previous experiences with MRI prostate imaging. Urologists and radiologists were apprehensive about the technique. Two major Australian trials commenced recruitment in 2012 and involved close collaboration between urologists and radiologists.
The ‘Sydney trial’13 was a negative predictive value study in which 150 men with suspicion of prostate cancer had an MRI followed by high-volume, trans-perineal biopsies. The biopsies were then correlated with the MRI. The trial reported a negative predictive value of more than 95%.
The ‘Brisbane trial’ went further. It compared an mpMRI + MRGB image-based diagnostic pathway with the current, standard TRUSGB biopsy-based pathway in 223 men with high or concerning PSA levels who had never had a prostate biopsy before. Quality control was provided by an expert radiological group in the Netherlands, who previously validated the PIRADS classification. The trial showed that using an image-guided diagnostic pathway for biopsy-naive men with raised or concerning PSA levels, and omitting high-volume, random biopsies, resulted in the following (PIRADS 3, 4 and 5 lesions were biopsied):
- The number of men needing any biopsy decreased by 51.1%.
- Diagnosis of low-risk cancer decreased by 89.4%.
- Diagnosis of intermediate- and high-risk cancer increased by 17.7%.
- The number of needles (biopsy cores) decreased by 87.9%.
The imaging strategy missed some intermediate- and high-risk cancers, but the TRUGB-biopsy strategy missed many more. The estimated sensitivity of the image-based pathway was 92%, with a negative predictive value of 96%. The estimated sensitivity of the biopsy-based pathway was 70%, with a negative predictive value of 71%. The true negative and positive predictive value, and the true specificity and sensitivity cannot be calculated in a diagnostic trial because it is unethical to perform a radical prostatectomy on a man with negative biopsies. The parameters are estimated using Bayes’ theorem.
The technology is not perfect. Negative tests require follow-up. There is now an evolving literature on the subject with comparable findings.
Future directions
MRI-based prostate imaging and targeted biopsies will reduce the documented harms of PSA testing to minimal levels without affecting morbidity or mortality. This is new knowledge and iatrogenic morbidity will decrease. ‘Prostate-specific anxiety’ will also decrease.
The reason for the unresolved debate over PSA testing for 25 years and community disquiet is that the wrong question – ‘should I have a PSA test?’ – has been asked for a generation. The real cause of the dilemma is, in retrospect, the standard urological protocol of high-volume, random prostate biopsies.
Currently, Australia has mpMRI quality-control and standardisation issues with image acquisition and interpretation. This is a matter of education for radiologists, MR radiographers, MRI machine manufacturers, information technology staff and urologists. This is occurring and there is a learning curve. There are some expert prostate MRI centres now. Expertise around the country is being acquired progressively.
Centres providing prostate mpMRI services will benefit from a targeted biopsy service where the biopsy needle position can be verified, with documented images in two planes. Therefore, a negative biopsy is validated as a true negative. This is the ideal quality control mechanism.
Imaging, even in the best centres, will miss around 5% of significant cancers. Follow-up of negative images and biopsies is still important. Prostate cancer is slow growing so a diagnostic delay of 6–12 months in this 5% group is highly unlikely to decrease patient longevity.
Cost-effectiveness of the image-guided pathway has been modelled and published.14,15 The image-guided pathway is cost-effective, improves quality of life and seems unlikely to ‘break the bank’. Additional cost savings also occur because of reduced procedural work. It is very unusual for a new medical intervention to increase quality-adjusted life years in association with cost saving to the community. The Australian Government is currently assessing the economic impact under the auspices of the Medical Services Advisory Committee.
How will this change GP practice in the future – risk stratification?
Patients with suspected prostate cancer now have the opportunity to use a powerful risk-stratification tool to assess their risk of significant or life-threatening prostate cancer. This will reduce anxiety, as an estimated half the patients with a high PSA will not need any biopsy.12 Men can also have a minimally invasive targeted biopsy using two needles per lesion.
GPs ordering an mpMRI should be appropriately cautious with a PIRADS 2 mpMRI report when there is a very high index of clinical and biochemical suspicion of significant cancer. There is still a definite but small place for high-volume, systematic biopsies in these cases, especially in the learning curve of a unit.
Before imaging was available, the most powerful risk stratification techniques were the high-volume, random and systematic biopsies. The following facts can be extracted from the recent literature to show how mpMRI/MRGB can be used as a risk stratification tool. The statistical device of AROC (area under the receiver operating curve), which is a computed index combining sensitivity and specificity is useful. A perfect test is 1.00.
- PSA = 0.56–0.5916,17
- PSA free-to-total ratio = 0.6318
- Prostate Health Index (PHI) = 0.7019
- Prostate cancer antigen 3 (PCA3) = 0.589–0.7120,21
- MRI/MRGB = 0.93622
It can be seen that a PSA test is just a little better than ‘a bet each way’ in risk-stratifying a man’s risk for significant prostate cancer. The PSA derivatives provide incremental improvement. However, there is a major increase in diagnostic power with the mpMRI/MRGB diagnostic pathway. The caveat here is that this diagnostic pathway requires quality control in the areas of image acquisition, image interpretation and targeted biopsy techniques.
Conclusion
Accurate anatomical and functional imaging of the prostate gland, and diagnosis of significant (intermediate- and high-risk) prostate cancer is now becoming available in Australia. This is a generational change and will lead to new diagnostic algorithms. There is also a learning curve in the implementation of this technology.
The functional aspects of the mpMRI imaging are a biomarker for selecting low-risk disease (non-life-threatening), and intermediate- and high-risk disease. This imaging is a very powerful risk-stratification tool.
Long-term longitudinal studies (up to 15 years) are required to validate the impact of the imaging diagnostic pathway. As with all tests, normal results need follow-up. Good collaboration between GPs, radiologists and urologists is essential.
Authors
Les C Thompson FRACS (Urol), Urologist, Wesley Hospital, Wesley Medical Centre, Brisbane, QLD. les.thompson@optusnet.com.au
Morgan R Pokorny FRACS (Urol) PhD, Urologist, Wesley Hospital, Wesley Medical Centre, Brisbane, QLD
Competing interests: None.
Provenance and peer review: Commissioned, externally peer reviewed.