Pharmacogenomics
PRACTICE POINT
There are international guidelines about potential uses of pharmacogenomic testing, and growing evidence from randomised controlled trials of the clinical utility for these tests to inform some prescribing decisions; their precise role in Australian general practice is still under consideration.
What is pharmacogenomics?
Genetic variations play a role in our ability to metabolise and respond to drugs, both in terms of efficacy and toxicity. Pharmacogenomic testing assesses the type of response a patient may have to a particular drug. Testing before prescribing medication can provide information about the likely effectiveness or risk of side effects for the patient.
What do I need to know?
The term ‘pharmacogenomics’ describes how common influence drug metabolism and response.
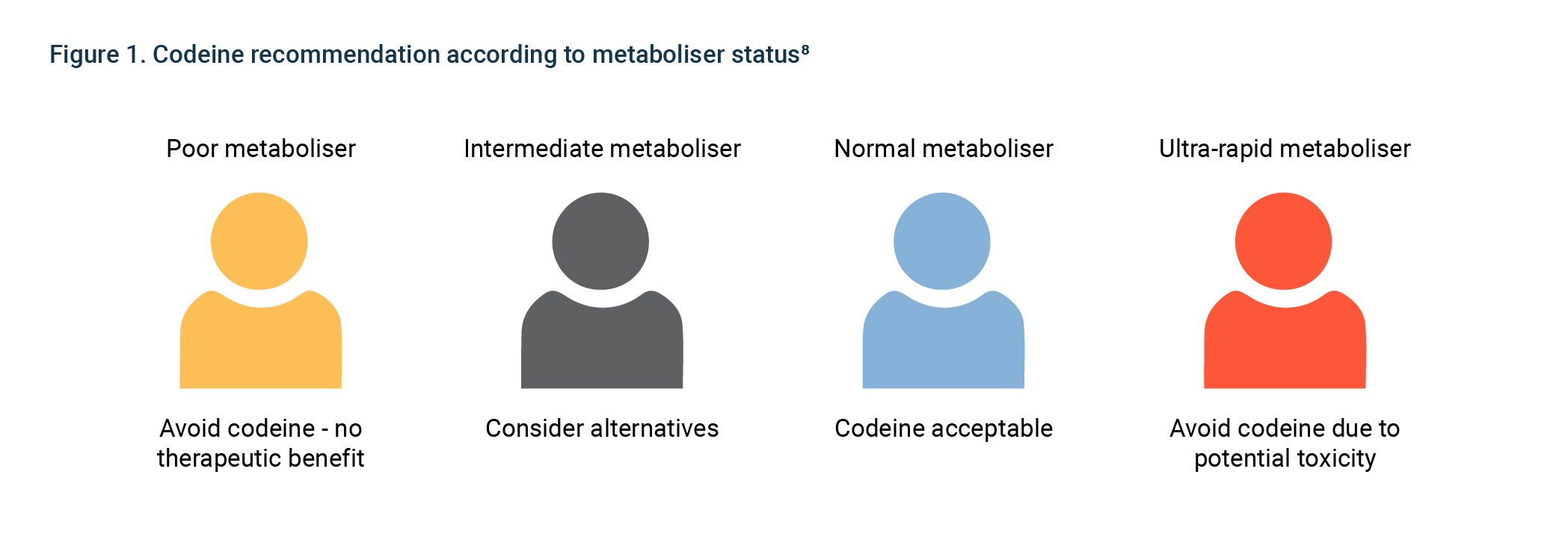
There are common variants in cytochrome P450 enzymes (CYPs) and drug receptors that influence the rate of metabolism of many commonly prescribed drugs. Individuals are typically classified as ‘poor’, ‘intermediate’, ‘extensive’ or ‘ultrarapid’ metabolisers depending on their CYP variants. Poor and ultrarapid metabolisers may require different dosages, or may be more susceptible to adverse drug effects.
Table 1 provides examples of drugs that may be affected by common gene variants.
Table 1. Examples of drugs affected by common
Gene name
|
Examples of drugs affected
|
Clinical consequence
|
|
CYP2D6 (5–10% of Caucasians are poor metabolisers; 1–2% of Caucasians are ultrarapid metabolisers)
|
Codeine
|
Poor metabolisers have no response to codeine; ultra- metabolisers are at an increased risk of side effects
|
| |
Selective serotonin reuptake inhibitors (SSRIs; eg paroxetine, fluvoxamine)
|
Ultrarapid metabolisers have no response to SSRI; poor metabolisers may need 50% lower dose
|
|
CYP2C19 (poor metabolisers 2–15%)
|
Clopidogrel
|
Poor metabolisers may require alternative anti-platelet therapy
|
|
VKORC1 and CYP2C9
|
Warfarin
|
CYP2C9 and VKORC1 genotypes may be useful in determining the optimal initial dose of warfarin
|
|
SLCO1B1
|
Simvastatin (not other statins)
|
Low-function genotype associated with increased risk of myopathy; consider alternative statin or lower dose
|
How genetic polymorphisms/variants affect drug metabolism
Genetic variation can affect:
- pharmacokinetics – how the drug metabolised by the body is affected by genetic variations in metabolising enzymes
- pharmacodynamics – what effect the drug has on the body is influenced by genetic variations in drug targets (eg receptors).
Pharmacogenomic testing analyses genes involved in these two processes.
One example is the cytochrome P450 multi-gene family, which produce enzymes involved in drug metabolism. P450 enzymes account for 70–80% of enzymes involved in drug metabolism. Common variations in P450 genes can affect the function of the enzymes produced, which in turn affects the metabolism of some drugs.
Genetic variations give rise to four different phenotypes in terms of drug response:
- Poor metabolisers who have markedly reduced or absent enzyme
- Intermediate metabolisers with reduced enzyme
- Extensive (or normal)
- Ultrarapid metabolisers who have high enzyme
Benefits of pharmacogenomics
The benefits of pharmacogenomic testing arise from the ability to tailor medication to the individual: specifically, to predict the correct dose to avoid toxicity or adverse events, and to know whether a particular drug will be effective in any given patient.
The benefits of pharmacogenomics include:
- achieving optimal drug doses quickly – the trial-and-error approach combined with repeated monitoring could be avoided
- minimising toxicity and adverse effects – knowledge of a patient’s genetic profile could reduce the likelihood of adverse outcomes and help direct clinicians towards suitable alternatives
- efficacious medications – genetic variations can predict which patients are likely to respond to certain medications, allowing clinicians to personalise treatment
- Evidence is accumulating from randomized controlled trials for some of these benefits for particular uses of pharmacogenomic testing (eg for antidepressants).
Limitations of pharmacogenomics
At present, there are several limitations of pharmacogenomic testing:
- Cost – there are currently only two pharmacogenetic tests funded through an MBS rebate: MBS item 73327 for the detection of genetic variants in the thiopurine S-methyltransferase gene for the prevention of dose-related toxicity during treatment with thiopurine drugs and MBS item 73323 for HLAB5701 testing prior to prescribing Abacavir therapy.
- Testing turnaround time – some results can take between five and ten working days to reach the clinician who ordered the In some cases, the trial-and-error approach to dosage would be completed within this timeframe.
- Evolving science – our understanding of how genetics influences our response to drugs is Confidence in the clinical utility of pharmacogenomic testing is slowly growing, but there is a need for more evidence about the cost-effectiveness of pharmacogenomic testing to inform specific prescribing decisions.
Genetic testing
Pharmacogenomic testing may be considered for patients with:
- significant side-effects from drugs for which pharmacogenomic variation in response is known (refer to Table 1)
- poor therapeutic response to specific medications
- potential suitability for using doses outside the usual range
Pharmacogenomic testing is not subsidised under the Medicare Benefits Schedule (MBS), but can be ordered by general practitioners through a number of commercial providers.
Further reading
- Bousman CA, Arandjelovic K, Mancuso SG, Eyre H, Dunlop BW. (2019). Pharmacogenetic tests and depressive symptom remission: A meta-analysis of randomized controlled trials. Pharmacogenomics, 20(1): 37-47
- Clinical Pharmacogenetics Implementation Consortium. CPIC guidelines. Stanford, CA: CPIC, 2014. [Accessed 6 September 2022].
- Roden DM, McLeod HL, Relling M, et al. Genomic Medicine 2. Pharmacogenomics. The Lancet, Volume 394, 2019, Pages 521-532
- Polasek T, Mina K, Suthers G. Pharmacogenomics in general practice. AJGP. Vol. 48, No. 3, Mar 2019: 100-105
- Rollinson V, Turder R, Pirmohamed M. Pharmacogenomics for Primary Care: An Overview. Genes 2020, 11, 1337; doi:10.3390/genes11111337
- Abbasi J. Getting pharmacogenomics into the clinic. JAMA 2016;316(15):1533–35.