Radiation therapy (RT) uses X-rays, discovered by Röentgen in 1895, to destroy cancer cells. Although breast conservation was first used in 1912 by inserting radium needles after surgical excision of the tumour, today, external beam radiation is produced using a linear accelerator. Conservation surgery (CS) removes macroscopic disease and a small margin of normal tissue. As residual microscopic disease is left behind in 40% of cases, often up to 4 cm from the primary site, RT is used to treat the remainder of the breast.1 The combination of CS and RT results in better cosmesis, is less complicated and associated with more positive body image than mastectomy, while achieving equivalent local tumour control and survival rates.2,3 Over the past 20 years, attempts have been made to avoid RT altogether after a lumpectomy, often by adding hormonal treatment (HT) such as tamoxifen, particularly for older patients with small oestrogen-receptor (ER)-positive, node-negative tumours. No consistent subgroup where RT can be avoided has been found, but trials are ongoing (Table 1).
Conservation techniques vary by surgeon, patient’s breast size, and location and size of the tumour. Various terms are used, including wide local excision (also called ‘lumpectomy’), where the tumour is removed with a small surgical margin, or larger excisions such as quadrantectomy.
A recent innovation for patients with larger breasts has been bilateral reduction mammoplasty. Here, excess fat, tissue and skin of both breasts are reduced to a proportional size for the patient’s body shape, and the breast shape is reconstructed by plastic surgery (Figures 1A, C).4–7 This approach reduces the amount of normal tissue that needs irradiation, and is associated with less retraction and better balance and symmetry after radiation. It is also less problematic than the marked imbalance from a unilateral mastectomy for patients with large breasts (Figure 1B).8
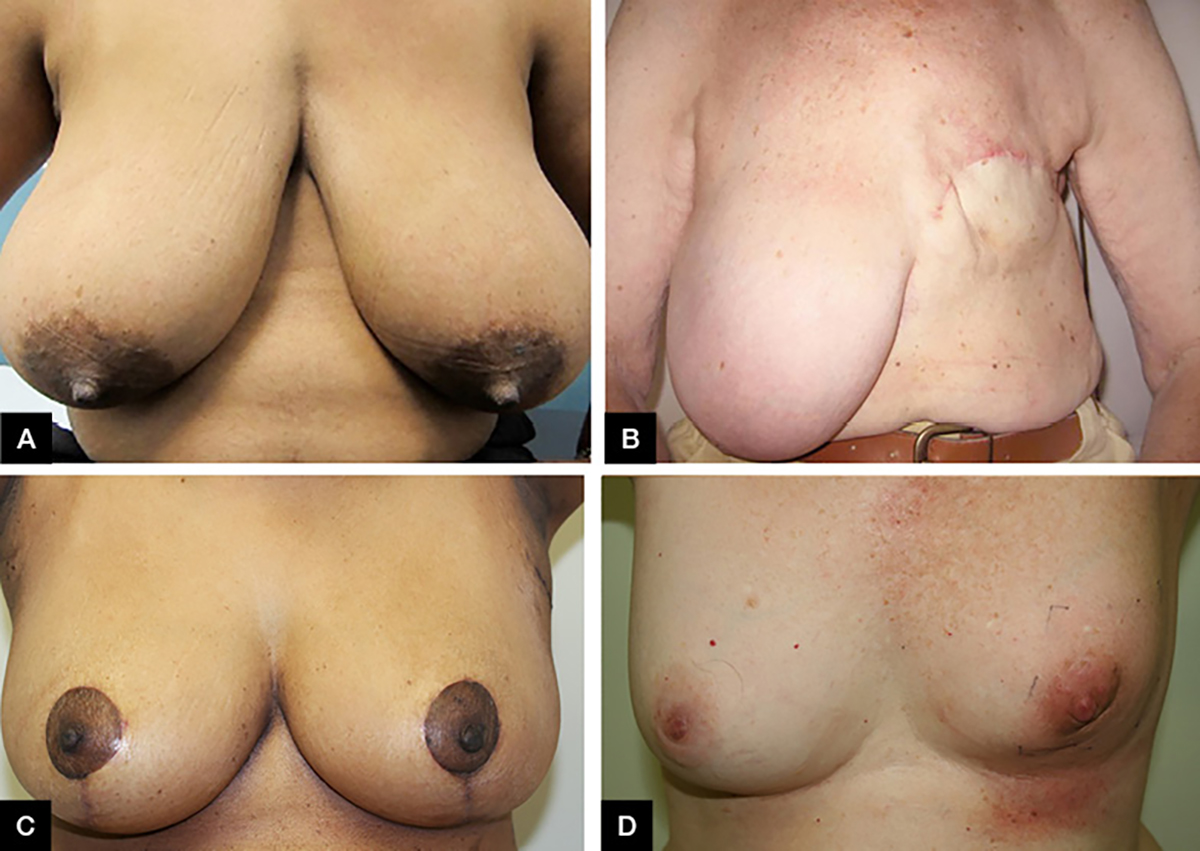
Figure 1. Breast surgical procedures
A. Original intact breasts of a patient with large breasts pre-operation; B. Woman with large breasts following a mastectomy; C. Patient from Figure 1A post-bilateral breast reduction mammoplasty; D. Skin erythema following five weeks of radiation therapy to the left breast and one week boost using electron beam
Table 1. Randomised trials of conservative surgery +/– radiation therapy +/– hormonal treatment
|
Author, publication date
|
Patientsn
|
Age ≥50 years (%)
|
≤20 mm(%)
|
Node-positive (%)
|
HT (%)
|
CT (%)
|
Median follow-up (years)
|
Recurrence in ipsilateral breast (%)
|
|
|
|
|---|
|
|
|
|
|
|
|
|
|
No RT
|
RT
|
RT+HT
|
HT
|
|
Clark,
1996
|
837
|
68
|
71
|
0
|
0
|
0
|
7.6
|
35.2
|
11.3
|
–
|
–
|
|
Forrest,
1996
|
589
|
73
|
43
|
23
|
73
|
26
|
5.7
|
24.5
|
5.8
|
–
|
–
|
|
Liljegren,
1999
|
381
|
61
|
100
|
0
|
0
|
0
|
8.8
|
24.0
|
8.5
|
–
|
–
|
|
Holli,
2001
|
152
|
73
|
100
|
0
|
0
|
0
|
6.7
|
18.1
|
7.5
|
–
|
–
|
|
Veronesi,
2001
|
579
|
56
|
84
|
31
|
12
|
17
|
9.1
|
23.5
|
5.8
|
–
|
–
|
|
Fisher,
2002
|
2163
|
60
|
55
|
40
|
Nil
|
40
|
20.7
|
39.2
|
14.3
|
–
|
–
|
|
Fisher,
2002
|
1000
|
80
|
98
|
0
|
67
|
0
|
7.2
|
16.5
|
4.8
|
2.8
|
16.5
|
|
Malmstrom,
2003
|
1187
|
81
|
91
|
0
|
7
|
2
|
5.1
|
14.0
|
4.0
|
–
|
–
|
|
Fyles,
2004
|
769
|
100
|
83
|
0
|
50
|
0
|
5.6
|
7.7
|
0.6
|
0.6
|
7.7
|
|
Hughes,
2004
|
636
|
100
|
100
|
0
|
50
|
0
|
12.6
|
4.4
|
0.6
|
0.6
|
4.4
|
|
Winzer,
2004
|
361
|
91
|
99
|
0
|
50
|
0
|
5.9
|
15.7
|
3.7
|
3.8
|
2.8
|
|
Ford,
2006
|
400
|
51
|
37
|
25
|
70
|
30.5
|
13.7
|
40.6
|
19.7
|
–
|
–
|
|
Potter,
2007
|
869
|
97
|
93
|
0
|
100
|
0
|
4.5
|
5.1
|
0.4
|
0.4
|
5.1
|
|
Blamey,
2013
|
1135
|
NS
|
100
|
0
|
50
|
0
|
13.9
|
10.2
|
3.9
|
3.9
|
10.2
|
|
Kunkler,
2015
|
1326
|
100
|
88
|
0
|
50
|
0
|
5
|
4.1
|
1.3
|
1.3
|
4.1
|
|
CT, chemotherapy; HT, hormonal therapy (usually tamoxifen); RT, radiation therapy
|
Studies of breast conservation
The landmark National Surgical Adjuvant Breast and Bowel Project (NSABP)-B06 study accelerated the uptake of CS and RT. Local recurrence rates at 20 years were almost 40% with lumpectomy alone versus 15% with CS and RT,9 and survival rates were equivalent to those after a mastectomy. Recent studies that evaluated omitting RT for older, lower risk patients found lower local recurrence rates, but patient subgroups that could avoid RT have not been identified (Table 1).10–24 For example, the NSABP-B21 randomised 1009 women with tumours <1 cm to RT, tamoxifen or RT and tamoxifen. Recurrence rates at eight years were 16.5%, 9.3% and 2.8% respectively, indicating that tamoxifen resistance occurs, but the combination of RT and tamoxifen is very effective. Nevertheless, avoiding RT could be considered for highly selected, older, well-informed patients with small, good-prognosis ER-positive tumours, but this is not currently considered best practice. A recently launched Australian and international randomised trial (EXamining PErsonalised Radiation Therapy for Low-risk Early Breast Cancer [EXPERT]) will address this question by comparing CS, RT and HT to CS and HT for women aged ≥50 years with small, low-grade breast cancer.25
The radiation therapy process
An initial assessment by a radiation oncologist is useful pre-operatively, but this mostly occurs following surgery. Following a decision to use RT, the option is explained in terms of the benefits and risks, duration, costs (mainly or totally covered by the Medicare Benefits Scheme [MBS]) and logistics. The decision to treat the breast alone, or some or all of the nodal regions, is also made. Following informed consent, a simulation session is performed with the patient in the treatment position, with measurements recorded by radiation therapists. Here, the breast and scars are marked with wire markers to outline important information on the subsequent inbuilt computed tomography (CT) scan (Figure 2A). Small, black tattoo dots are placed beneath the patient’s skin with a fine needle to accurately reproduce the patient’s position on daily treatment.
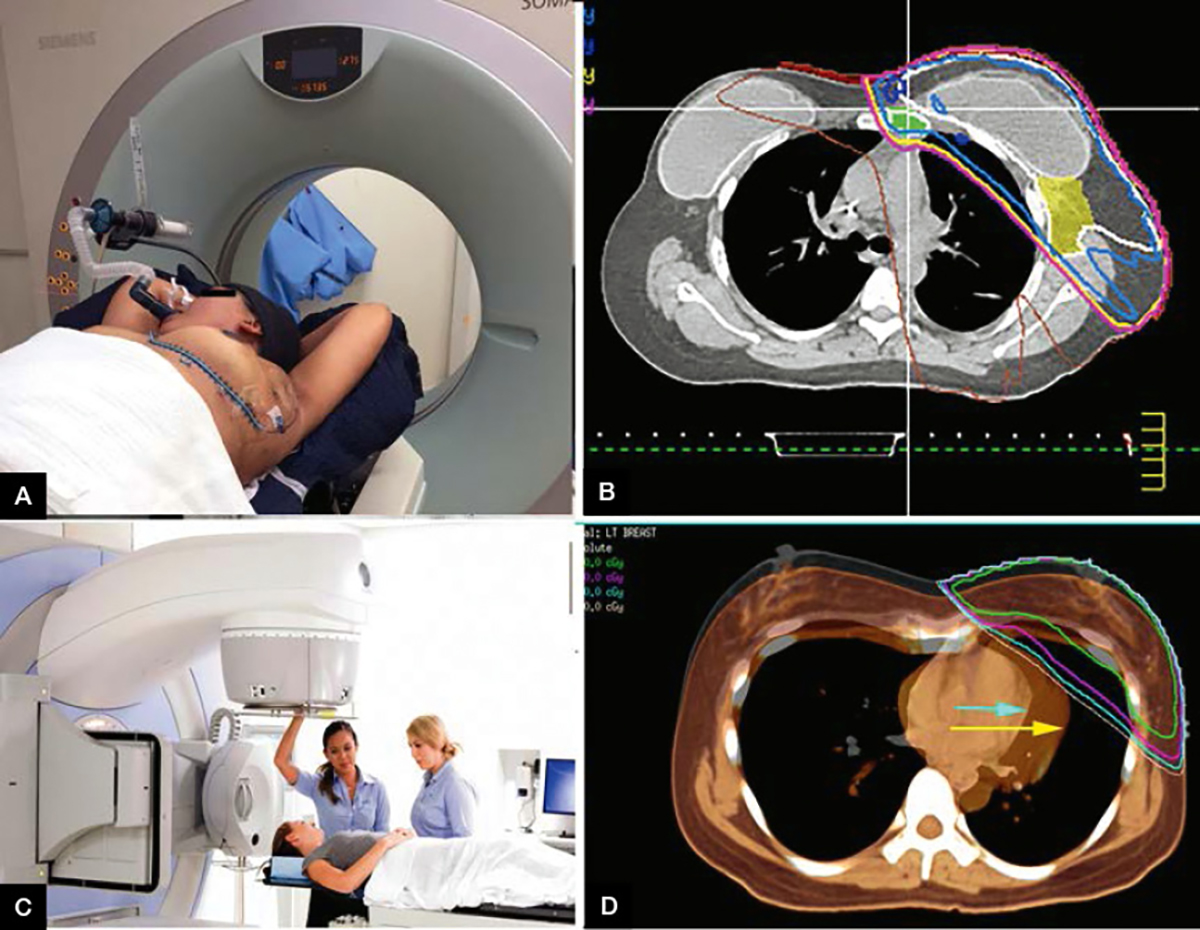
Figure 2. The radiation therapy process
A. Photograph of deep inspiration breath hold (DIBH) setup with mouthpiece; B. Axial slice from planning computed tomography (CT) of implant and radiation dose lines conforming to the target IMC and breast tissue indicated in purple; C. Elekta Synergy – Linear accelerator used in external beam radiotherapy treatment; D. Axial slice from planning CT in Pinnacle planning software for DIBH. The orange scan represents the heart position during free-breathing (yellow arrow) and the black-white underlay shows the heart’s position in breath-hold full inspiration (aqua arrow)
The CT data are used to plan an individualised three-dimensional (3D) RT treatment that is appropriate for that patient. Using 3D planning software, it is possible to plan a treatment that accurately delivers the prescribed dose and fractionation to the target tissue (outlined by the radiation oncologist using previous imaging, histology reports and clips placed at surgery), while minimising the dose to the surrounding healthy tissue, including the heart, lungs and contralateral breast (Figure 2B).
Following approval of the radiation plan by the radiation oncologist, the data are linked to treatment software of the treatment machine (linear accelerator). It is then ready for the patient to start therapy, usually daily for nine or 10 times per fortnight (Figure 2C). In the head of the gantry are multi-leaf collimators (MLCs), which shield out the radiation to manipulate the dose to deliver the plan. MLCs are tiny rectangular pieces of tungsten, which move per the computer-generated plan to influence the dose distribution, like a camera aperture. Traditionally, a course of RT to the whole breast would be a dose of 50 Gy in 2 Gy fractions (attendances), usually followed by a 10–16 Gy boost dose to the tumour bed, the most common place for a breast cancer recurrence. A boost dose is often delivered via an electron beam (Figure 1D) for superficial tumours, or a photon beam. Current practice variation in Australia has been reported elsewhere.26
The European Organisation for Research and Treatment of Cancer (EORTC) in 2016 examined 5569 women with early-stage breast cancer who were treated with CS and RT with or without a boost. Their results showed that the boost reduced the 20-year local recurrence from 17% to 12% (P <0.001) for all patients. For patients with hormone receptor–negative, high-grade tumours, the boost reduced the breast recurrence rate from 31% to 5% (P = 0.01).
Patients are usually reviewed weekly during treatment to assess progress by the radiation therapists, radiation oncologist and/or nurses. Common side effects are listed in Table 24–7 and Figure 1D. At the completion of the radiation course, a follow-up consultation by the radiation oncologist or other member of the multidisciplinary team will usually take place six to eight weeks post-treatment to assess physical and psychological health.
Table 2. Potential side effects of radiation that are not uncommon4–7
|
Timing
|
Symptoms
|
Incidence
|
Signs
|
Treatment
|
|---|
|
Acute
|
Skin redness/itch
|
>50%
6–10%
|
Erythema
Dry desquamation
Moist desquamation
|
Vitamin E, sorbolene, aloe vera, MooGoo
Consider hydrocortisone 1% for itchy areas
Avoid wearing tight clothing (eg bras and collars)
Wash the skin gently with warm water and a mild, unscented soap; rinse well and pat dry gently
Avoid using aluminium-based deodorants
Avoid wet shaving within the treatment field
For moist desquamation (usually week four or five) use Solugel or Paw Paw cream. Sometimes silver sulfadiazine is recommended following treatment
|
|
|
Swelling of breast
|
12.6%
|
Breast oedema
|
Keep moisturised. If acute redness occurs after post-radiation erythema has resolved, this is usually cellulitis that requires antibiotics
|
|
|
Sore throat
|
–
|
Nil specific
|
May occur in weeks three to four if radiation is given to the supraclavicular fossa. Paracetamol syrup
Eat soft foods and avoid hot and spicy foods
|
|
Sub-acute
|
Pain in breast
|
10–50%
|
Mild shrinkage
|
Rub moisturising cream (eg sorbolene, vitamin E)
into site
Can be scar-related pain or costochondritis
Check vitamin D levels
|
|
|
Dry cough, fatigue, shortness of breath six weeks to six months after treatment
|
1–2%
|
Nil specific
|
X-ray or high resolution computed tomography (CT) scan of chest shows changes inside and sometimes outside the treatment field
Bronchodilators and oral steroids and antibiotics are often used
|
|
|
Transient swelling following exercise or physical activity
Pain, heaviness in the affected limb
|
Mild: 6–10%
Severe: 1–5%
|
Lymphoedema
|
Weight management
Encourage early detection using bioimpedance devices such as L-Dex
Consider referral to appropriately qualified lymphoedema practitioner (www.lymphoedema.org.au)
|
New radiation techniques
New techniques in RT make it possible to maximise clinical benefit and minimise toxicity. With 3D planning software, heart and lung doses can be significantly reduced, minimising cardiac and lung toxicity. Deep inspiration breath-hold (DIBH) is a new technique predominantly used in the treatment of left-sided breast cancers. One example of this system uses the Elekta Active Breathing Coordinator (ABC) solution, whereby using a snorkel-shaped mouthpiece and gating system, the patient can be treated only when in full inspiration, which pushes the heart posteriorly and inferiorly away from the chest wall27 (Figure 2A, B, D). This results in lower heart and coronary artery dose, with less heart toxicity, and assists with daily reproducibility by eliminating the movement of free breathing.28
Accelerated partial breast irradiation (APBI) is a localised form of radiation given over one or two weeks. One technique is brachytherapy, where radioactive sources are inserted in the tumour bed following a lumpectomy. An alternative technique is a single electron beam dose given to the cavity in the operating theatre.29 Another involves a short course of five large treatments of 6 Gy over a two-week period using 3D conformal radiation or modulated techniques.30 There is debate as to which patients would benefit from this form of radiation therapy as it is known that recurrences can develop outside the primary tumour site.21 Clinical guidelines for the use of this technique outside clinical trials were updated in 2016.31
Shorter courses of RT, known as hypofractionation, have been studied because of the cost and inconvenience of five to six weeks of treatment. Hypofractionation involves the delivery of higher daily doses of radiation in a smaller number of fractions, with a lower overall dose that is biologically equivalent to a standard dose. A common regimen, proven to have comparable outcomes, is 40.05–42.5 Gy in 15–16 fractions over about three weeks.30,32,33 A recent Australia-wide campaign (Choosing Wisely Australia) by The Royal Australian and New Zealand College of Radiologists (RANZCR) advised ‘Don’t initiate whole-breast radiation therapy as a part of breast conservation therapy in women aged ≥50 years with early-stage invasive breast cancer without considering shorter treatment schedules’.34
Intensity-modulated radiation therapy (IMRT) is a form of external beam radiation therapy whereby the head of the linear accelerator (gantry) can modulate the dose of radiation being delivered while the beam is on. This is made possible by using MLCs in the head of the gantry, which allows segmental blocking capabilities to modulate the shape of the beam.
It is also possible for the gantry to move simultaneously with the MLCs to provide even greater flexibility in treatment delivery and dose rate. This is known as volumetric-modulated arc therapy (VMAT). The plan is displayed using isodose lines, which define areas receiving the same radiation dose. Areas of low, prescribed and high dose can be visualised similarly to isobars on a weather map. Figure 2D shows the isodose lines on the axial views of a VMAT DIBH plan. This technique requires the use of inverse planning systems, whereby objectives or goals required for the plan are prioritised so that a computer algorithm can create the plan that best fulfils them.
These new technologies can reduce the dose to surrounding healthy organs, and produce highly conforming dose distributions and homogeneity around the target area; more normal tissue receives a low dose of radiation. It should also be noted that this may decrease rates of dermatitis, acute moist desquamation (red, exposed dermis and serous oozing), breast oedema and breast fibrosis.35
Conclusion
The CS and RT combination is an effective treatment method for early breast cancer and has been recommended in consensus guidelines for more than 20 years.36 This combination approach has been shown to be equivalent in survival and recurrence rates to mastectomy, minimising the need for extensive surgery. New technologies in RT have made it possible to further individualise treatment plans, reduce normal tissue toxicity with the aim of further reducing local recurrence rates, and improve survival. Ongoing studies are exploring which patients could avoid RT after CS.
Authors
Ellen Tailby BSc-Psych, MMedRadSc, Radiation Therapist, Macquarie University Hospital Sydney, Radiation Oncology, Radiation Oncology Associates and Genesis Cancer Care Pty Ltd, Sydney, NSW
John Boyages AM MBBS (Hons), FRANZCR, PhD, Professor of Breast Oncology, Macquarie University Hospital Sydney, Radiation Oncology, Radiation Oncology Associates and Genesis Cancer Care Pty Ltd, Sydney, NSW; Department of Clinical Medicine, Faculty of Medicine and Health Sciences, Macquarie University, Sydney, NSW. john.boyages@mq.edu.au
Competing interests: None.
Provenance and peer review: Not commissioned, externally peer reviewed.
Acknowledgments
The authors thank the following people and organisations that helped with the production of this manuscript. Lesley Baker and Sergio Duque for planning patient involved in Figure 2; Genesis Cancer Care for Figures 2A–D; BC Publishing (Boyages, Breast cancer: Taking control); Philippa Sutton for editing and manuscript management.