Approximately 13,750 refugees come to Australia from resource-poor and war-torn countries each year.1 Recent national and international studies have reported high levels of vitamin B12 (B12) deficiency in newly arrived refugees, particularly those from Bhutan, Afghanistan and Iran.2,3 B12 deficiency is associated with depression, fatigue, paraesthesia, memory problems and macrocytosis.4 Levels below 150 pmol/L (203 pg/ml) are classified as low and can cause permanent neurological damage and fetal abnormalities in babies of untreated mothers.5,6 Levels of 151–240 pmol/L are considered borderline deficiency and may be associated with depression and cognitive decline.7 B12 deficiency is associated with neuropsychiatric symptoms and may be of particular relevance to refugees, many of whom have a background of psychological ill-health related to torture and trauma.
B12 deficiency in newly arrived refugees has been attributed to a lack of animal-source foods in the country of origin and intestinal parasites (eg Helicobacter pylori).8–10 Despite evidence that food insecurity can continue to be a problem after arrival,11 it is often assumed that B12 levels will improve after arrival in a country where animal-source foods such as meat, eggs and milk are more readily available, and intestinal parasites have been eliminated.12 However, this assumption has never been tested.
There is currently little evidence to guide practitioners on the length of treatment for newly arrived refugees who are B12 deficient, or whether those with borderline levels should be followed up. This study aimed to document the association between B12 levels and neuropsychiatric symptoms, and to describe changes in the levels after resettlement in a cohort of refugees.
Methods
Study population
All newly arrived refugees over the age of 18 years who attended the Migrant Health Service in Adelaide, South Australia, between October 2011 and November 2012 were enrolled (ascertainment, 100%) in the study. Their countries of origin were Bhutan, Iran, Afghanistan and the Horn of Africa (Ethiopia and Eritrea). Approximately 50% of the 1300 refugees who come to South Australia each year have their initial screening and early follow-up at the Migrant Health Service. The demographic profile of newly arrived refugees seen at the Migrant Health Service was similar to the overall demographic profile of refugees being resettled in South Australia.
Study design
Data were collected at baseline (initial screening) and again at 4–8 months after the initial screening. The mean time between arrival and screening was 15 days (range = 2–41 days). The mean time between baseline testing and follow-up was 5 months (range = 4–8 months).
Baseline data
All participants were tested for vitamin B12, folate, haematological indices, iron studies, intestinal parasites and Helicobacter pylori stool antigen as part of the routine screening. Participants were asked to complete a 16-item questionnaire using a 5-point Likert scale to address neuropsychological symptoms in the preceeding 4 weeks. The questionnaire comprised the Kessler Psychological Distress Scale (K10),13 the general health question from the Australian Bureau of Statistics’ National Health Survey14 and five questions on relevant symptoms that were developed by the team (‘in the last 4 weeks how often did you have (1) problems with your memory; (2) feel irritable; (3) have strange sensations in your arms or legs (numbness, tingling or burning); (4) have a decreased appetite; (5) feel dizzy?’) The K10 has been validated for participants who speak Farsi (Persian).15 The questionnaire was translated into Farsi and Nepali (the language of refugees from Bhutan) by accredited translators and back-translated by GG and HH. An accredited interpreter who spoke Amharic and Tigrinya was used for patients from the Horn of Africa. The questionnaire was developed in consultation with community leaders. All patients gave verbal, informed consent interpreted from English by the interpreter or health worker.
Management of B12 deficiency
B12 deficiency was defined as ≤150 pmol/L, borderline as 151–240 pmol/L and normal as >240 pmol/L. Deficiency was managed at the discretion of the general practitioner (GP). Most participants with B12 levels ≤150 pmol/L were treated with intramuscular B12; those with borderline levels at baseline were given dietary advice, but not injections.
Follow-up study
Patients whose B12 levels were ≤240 pmol/L at baseline were retested as part of the clinical follow-up. All participants were invited to repeat the baseline questionnaire irrespective of their B12 levels.
Analysis
Chi square analysis was performed on dichotomous variables. Paired t-test analysis was used for before-and-after comparisons of continuous data. Two-sample t-tests and analysis of variance (ANOVA) were used to compare independent samples with unequal variances at one point in time for baseline or follow-up. The Kruskal-Wallis test was used to make comparisons for variables that were not normally distributed. The K10 data were scored to a total of 50. Responses were collapsed to dichotomous variables to compare responses for each of the five neurological symptoms across the three domains of B12. Scores of 2–5 indicated the presence of a symptom, while a score of 1 indicates absence. The interpretation of the scores was in accordance with community advice.
This study was approved by the Human Research Ethics Committees of South Australian Health and The University of Adelaide (H-188-2011).
Results
Newly arrived refugees aged over 18 years during the study period (n = 136) had the initial screening blood test (63 women, 73 men). They were from Bhutan, Iran, Afghanistan and the Horn of Africa (Table 1). The mean age of participants was 36 years (SD = 12.2) for women and 39 years (SD = 17.6) for men.
Table 1. Distribution of vitamin B12 results for participants from different countries at baseline
|
|
Origin
|
n
|
% Male (95% CI)
|
Mean age (SD)
|
Vitamin B12 levels
|
|---|
|
|
|
|
|
Deficient B12
(≤150 pmol/L)
n = 21
n (%)
|
Borderline B12
(151–240 pmol/L)
n = 64
n (%)
|
Normal B12
(>240 pmol/L)
n = 50
n (%)
|
|
Bhutan
|
92
|
54 (44–64)
|
40 (16.5)
|
13 (14)
|
52 (56)
|
27 (29)
|
|
Afghanistan
|
30
|
60 (42–77)
|
30 (10.8)
|
7 (23)
|
7 (23)
|
16 (53)
|
|
Horn of Africa
|
12
|
25 (0.5–49)
|
34 (9.2)
|
1 (8.3)
|
5 (42)
|
6 (50)
|
|
Iran
|
1
|
0
|
NS
|
0 (0)
|
0 (0)
|
1
|
|
Total
|
135*
|
|
|
|
|
|
|
*Country of origin for one participant was unknown; NS, not specified
|
Figure 1 outlines the stages and response rates arranged by B12 levels. The overall response rate for the follow-up blood test among participants with low and borderline B12 levels was 95%. Of the total baseline population, 68% completed the follow-up questionnaires. There was no significant difference in the response rates of those with low, borderline or normal B12 levels, or between people from different countries of origin.
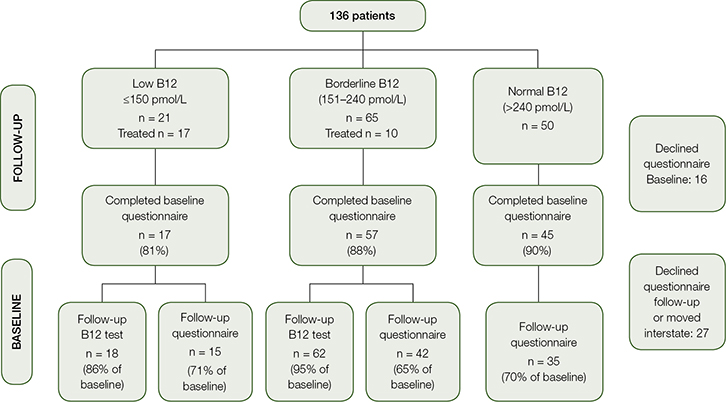 |
| Figure 1. Flow chart illustrating response rates at baseline and follow up |
Baseline screening results
Participants’ mean B12 level on arrival was 236 pmol/L (SD = 47); 21 participants (15.5%) were deficient and 65 (48%) had borderline levels of B12. Men were more likely to be deficient in B12 than women. No association was found between B12 level and red cell size, serum iron, mean K10 score or H. pylori stool antigen (Table 2). No participant with B12 deficiency tested positive for coeliac disease, parietal cell or intrinsic factor antibodies.
Table 2: Haematological indices, ferritin, folate, K10 scores and rates of H. pylori stool antigen for different vitamin
B12 levels at baseline
|
|
|
B12 deficiency
|
Borderline B12
|
Normal B12
|
Reference range
|
|---|
|
|
(≤150 pmol)
n = 21
|
(151–240 pmol/L)
n = 65
|
(>240 pmol/L)
n = 50
|
|
|
Mean corpuscular volume (SD)
|
86.4 fL
(5.42)
|
86.4 fL
(5.64)
|
85.6 fL
(5.86)
|
80–96 fL*
|
|
Mean haemoglobin (SD)
|
151 g/L
(15.6)
|
144 g/L
(15.4)
|
144 g/L
(17.4)
|
130–185 g/L*
|
|
Median female serum ferritin
n = 63
(IQ range)
|
26 mg/L
(22–30)
|
29 mg/L
(17–48)
|
64 mg/L
(19–175)
|
30–150 mg/L†
|
|
Median male serum ferritin
n = 63
(IQ range)
|
118 mg/L
(56–205)
|
99.5 mg/L
(75–137)
|
92 mg/L
(25–157)
|
40–260 mg/L
|
|
Mean red cell folate (SD)
|
22.4 nmol/L
(9.87)
|
19.9 nmol/L
(9.36)
|
20.4 nmol/L
(9.74)
|
7–40 nmol/L*
|
|
Number with positive stool antigen for H. pylori (%)
|
6
(28.6%)
|
17
(26.5%)
|
9
(18%)
|
|
|
Mean K10 score (SD)
|
18.9
(6.65)
|
21.7
(7.95)
|
18.9
(6.57)
|
Levels of distress:16
5–15 = Low
16–21 = Moderate
22–29 = High
30–50 = Very high
|
|
*Reference range used by testing laboratory: females 115–155 g/L and males 135–175 g/L; †rates in females refers to menstruating females
|
Neurological symptoms
In the questionnaire, 12.6% of participants scored >30 on K-10, which indicated severe distress. Baseline symptoms of fatigue, irritability and paraesthesiae were distributed across all B12 groups. Participants from Bhutan with B12 deficiency were most likely to describe the experience of peripheral neuropathy (8 of the 13 Bhutanese who had levels <150 pmol/L).
Follow-up
At follow-up, 17 of the 21 participants with B12 deficiency received intramuscular B12 (three moved to another city and one declined). Of the 65 participants with borderline B12 level, 10 were treated with intramuscular B12 at the doctor’s discretion. Treatment elevated B12 levels from 149 pmol/L (SD = 19.8) before supplementation to 514 pmol/L (SD = 97.6; P <0.0001). Of the participants with borderline B12 level (mean baseline = 197 pmol/L [SD = 12.7]) who did not receive intramuscular supplementation (54/65), six (11%) became frankly deficient at follow up and 64% continued to have borderline levels (211 pmol/L [SD 67.2], P =13).
At follow-up, severe distress (K10 scores >30) was present in 24% of all participants. It was found during the follow-up that 18% of the total cohort were receiving counselling and/or were on antidepressant treatment. There was no significant difference in K10 scores for patients with low, borderline or normal B12 levels, either at baseline or follow-up.
Treatment of B12 deficiency did not result in improvements in neurological symptoms. Figure 2 shows that rates of fatigue remained high at 3 months for those who had low, borderline and normal B12 rates at baseline. Fatigue persisted even among the groups treated for B12 deficiency. Similar results can be seen for irritability (Figure 3) and paraesthesia (Figure 4), which were reported at higher rates during follow-up, even in those with B12 deficiency who had been treated.
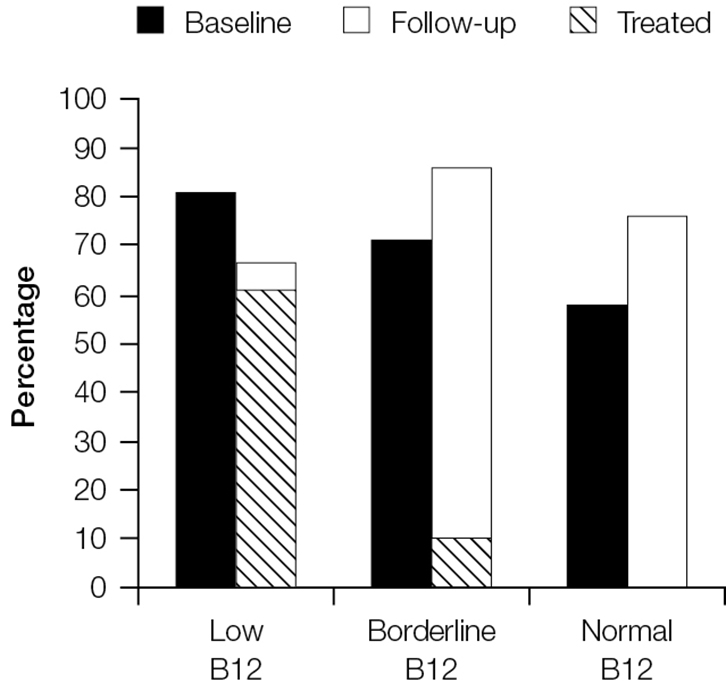 |
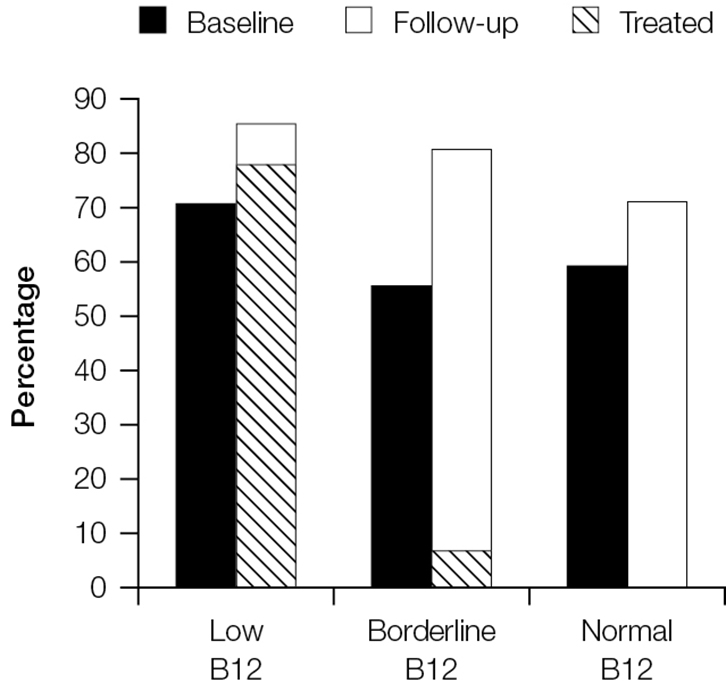 |
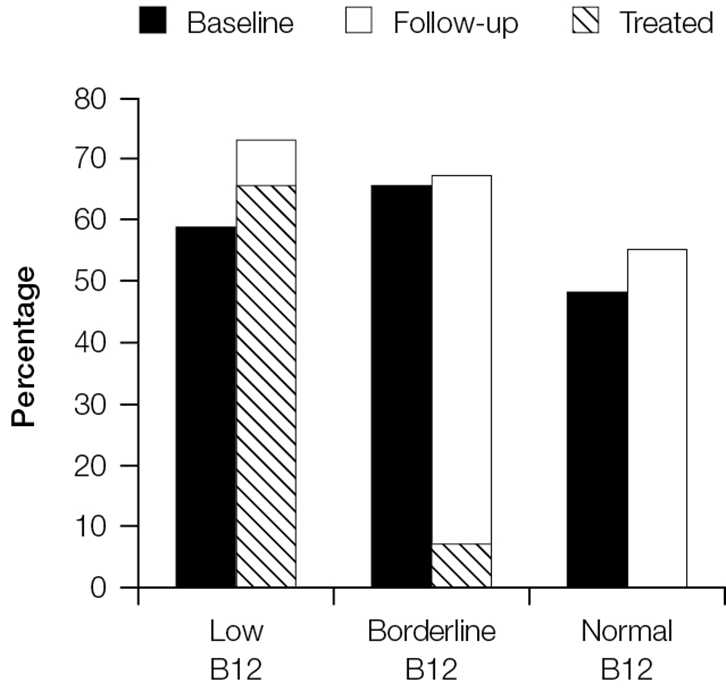 |
Figure 2. Proportion of patients reporting fatigue at baseline and follow-up stratified by vitamin B12 group
The cross-hatched section shows the proportion of patients in each category who were treated and reported fatigue at follow-up |
Figure 3. Proportion of patients reporting irritability at baseline and follow-up stratified by vitamin B12 group
The cross-hatched section shows the proportion of patients in each category who were treated and reported irritability at follow-up |
Figure 4. Proportion of patients reporting paraesthesiae at baseline and follow-up stratified by vitamin B12 group
The cross-hatched section shows the proportion of patients in each category who were treated and reported paraesthesiae at follow-up |
Discussion
Health practitioners are that taught vitamin B12 deficiency is a rare condition, and that the intake of animal-source foods in the absence of particular pathologies should ensure adequate levels. Current teaching also suggests that B12 deficiency can be identified through symptoms or blood parameters. This study has found these assumptions to be inaccurate for newly arrived refugees. There is no significant association between B12 levels and any blood parameters (eg macrocytosis), neurological symptoms or psychological symptoms (as measured by K10). In this study, men were more likely to have B12 deficiency, which is consistent with an earlier multicentre study.2
Most practitioners do not follow-up borderline B12 levels because they are not reported to be clinically significant,4 and it is thought that levels will improve after settlement.12 It has been assumed that B12 levels are low in refugees because of decreased intake of animal-source foods in the country of origin.12 This study found that patients with borderline B12 levels did not experience an increase in their levels after arriving in Australia and some even developed deficiency. Our study suggests that patients with borderline B12 should be monitored and/or treated.
The reasons for the failure of B12 levels to improve after settlement are likely to be complex and multifactorial. They may include ongoing food insecurity,11 untreated intestinal parasites and genetic factors. Our study was underpowered to test the association between H. pylori infection and B12 deficiency, but the prevalence in patients with low and borderline levels was higher than those with normal B12 levels.
There are several limitations to this study. The experience of resettling in Australia brings its own psychological burden. The background psychological stress experienced by participants may have exceeded the more subtle psychological symptoms associated with B12 deficiency. This may result in a homogenising of the questionnaire results on psychological symptoms across the three B12 groups. As the symptoms of irritability increased in all groups at 3 months, irrespective of whether they received B12 supplements, this indicates some of the background psychological stress. However, as the mean level of expressed distress using the K10 questions remained in the moderate level the resilience of refugees may be a protective factor.
The questionnaire itself presented its own difficulties. Many respondents struggled with answering the questionnaire. Most questions generated a long narrative rather than a direct answer. Respondents from some countries were unfamiliar with the Australian approach where doctors asked questions rather than simply provide instructions. Grading answers on the Likert scale and describing peripheral neuropathy symptoms proved to be especially challenging. It is also important to note that only the Bhutanese have a local term (jham-jham) that could be used to delineate peripheral neuropathy symptoms.
Implications for general practice
High rates of low B12 levels in newly arrived refugees is a recent finding. It has implications for their health, resettlement and future in Australia, including the health of the children with B12-deficient mothers.
This study provides new information on the ongoing management of B12 deficiency in this population group. GPs cannot rely on symptoms or macrocytosis to determine which patient needs testing for B12 deficiency, nor can these clinical parameters be used for follow-up when results are low or borderline. Similarly, it cannot be assumed that borderline levels will improve once newly arrived refugees have better access to animal-source foods. For now it would appear prudent to proactively follow-up those with low or borderline B12 levels for at least 6 months.
Further studies will be needed to determine the length of time for follow-up of those with low and borderline B12 levels. This study demonstrates that the answers are not straightforward. Assumptions regarding the current management, including follow-up of B12 deficiency in the general community, may also need to be challenged.
Authors
Jill Benson AM MBBS, DCH, FACPsychMed, MPH, Director, Health in Human Diversity unit, Discipline of General Practice, University of Adelaide, Adelaide, SA. jill.benson@adelaide.edu.au
Christine B Phillips MBBS, MA, MPH, FRACGP, ACT Refugee Health Service; Associate Professor, Academic Unit of General Practice and Community Health, Medical School, Australian National University, Canberra, ACT
Margaret Kay MBBS(Hons), PhD, FRACGP, DipRACOG, Senior Lecturer, Discipline of General Practice, The University of Queensland, Herston, QLD
Hoda Hanifi, BNurs, Postgrad Dip Mental Health Nursing, Clinical Nurse, Migrant Health Service, Adelaide, SA
Gauri Giri, MNurs, BSc (Hons), Nurs, Community Health Worker, Migrant Health Service, Adelaide, SA
Catherine Leahy BMus/BArts (Hons Psychology), PhD Medicine, Dip Counselling, Research Academic, Health Sciences, University of Adelaide, Adelaide, SA
Michelle Lorimer BSc (Math & Comp Sci)(Hons), Senior Statistician, School of Population Health, University of Adelaide, Adelaide, SA
Competing interests: None.
Provenance and peer review: Not commissioned, externally peer reviewed.
Acknowledgement
This research was funded through a Primary Health Care Research, Evaluation and Development (PHCRED) Grant.