Cancer of unknown primary (CUP) site is defined as metastatic cancer with no known site of origin. Other terms include ‘occult’ primary tumour. CUP is heterogeneous in clinical presentation, histopathology (where available), response to therapy and prognosis.1 CUP is vastly under-researched relative to its burden and, despite being relatively common, public awareness is very low.
Burden of disease
In 1982–2010, CUP was one of Australia’s top 10 most commonly registered cancers and causes of cancer death. After occurring at a stable rate in 1982–97, the incidence of CUP in Australia has declined from 21.3 and 14.4 per 100,000 men and women respectively in 1997, to 13.4 and 9.2 per 100,000 respectively in 2011 (Figure 1). In 2011, CUP was the 12th most common cancer, affecting 1 in 68 people by the age of 85 years.2 A declining incidence has been observed in developed countries, and this has been attributed mainly to advances in diagnostic techniques. CUP mortality rates have also declined over time, but the burden remains very high and most patients have a poor prognosis.2 Mortality rates decreased from 14.6 and 10.4 per 100,000 men and women respectively in 1997, to 10.8 and 7.4 per 100,000 respectively in 2011 (Figure 1). In 2011, CUP was the fifth most common cause of cancer death in Australia and the 5-year relative survival rate was 13.8% (95% CI, 13.2–14.5).2
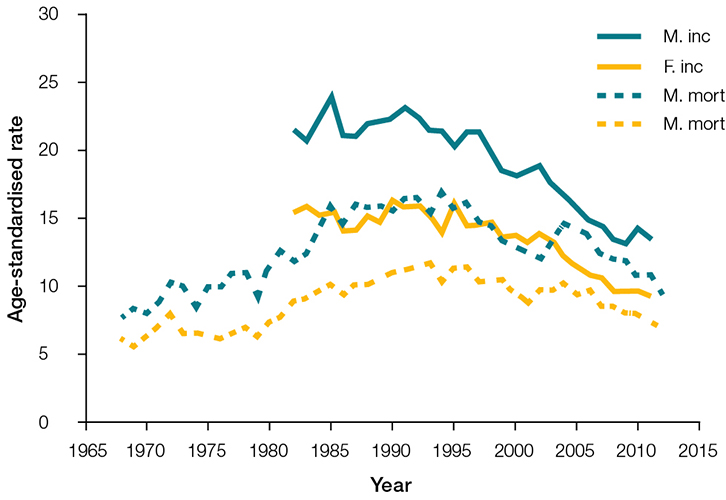 |
| Figure 1. Age-standardised incidence and mortality rates by year28 |
Three CUP subtypes have been identified by the National Institute for Health and Care Excellence (NICE) on the basis of increasing levels of confidence in the diagnosis of cancer.3 In the first category (‘malignancy of unknown origin’), patients are too unwell to undergo tissue sampling because of metastatic disease and/or comorbid illness. Thus, the diagnosis is made on a clinical basis only. In the second category (‘provisional CUP’), patients undergo at least initial diagnostic investigations. Metastatic cancer is confirmed cytologically or histologically, but the origin remains unknown. In the third category (‘confirmed CUP’), metastatic cancer is confirmed histopathologically and all appropriate specialised investigations fail to identify the site of origin. The incidence of the CUP subtypes is unknown, but the ‘confirmed CUP’ category is believed to be rare. Among the elderly, however, as many as 50% may be categorised as having ‘malignancy of unknown origin’.4
Certain subgroups of the population are at greater risk of CUP. The strongest risk factor is age: CUP is vanishingly rare in people under the age of 40 years and in Australia in 2011 the average age at diagnosis was 75 years.2 CUP occurs at higher rates in men than in women; in people living in remote areas, compared with those in major cities; in people living in areas of lower socioeconomic status, compared with higher; and in Aboriginal and Torres Strait Islander peoples, compared with non-Indigenous Australians.5–8 These data suggest that reduced access or delayed presentation to medical services is related to a CUP diagnosis.
Surprisingly, little is known about lifestyle and other risk factors for CUP. Evidence from a single prospective cohort study suggests that heavy smoking is associated with an increased risk of CUP (about 4-fold), and high alcohol consumption and wide waist circumference may also increase risk.9 However, this analysis included all CUP subtypes, potentially masking aetiologically relevant associations for the minority subtypes, confirmed and provisional CUP. In another cohort study, the strongest association with smoking was observed in patients who presented with lung metastases.10 The authors hypothesised that many of these CUP cases may be undetected primary lung cancers.
Diagnosis
Although the pathogenesis of CUP is poorly understood, confirmed CUP is believed to have a unique natural history, defined by early dissemination and a short history (<3 months) of symptoms and signs.11 Data from the Commonwealth Department of Veterans’ Affairs (DVA)12 on clients (median age 82 years) supports international evidence13 that the presenting symptoms for CUP can be non-specific and vague (eg weight loss and pain). In this Australian study, patients with CUP had significantly more comorbidities before diagnosis than patients with metastatic cancer of known primary site. Interestingly, there was no difference in the rate of general practice consultations in the 3 months prior to diagnosis for these patient groups.12 However, patients with CUP were more likely to present to an emergency department,12 mirroring findings from the Routes to Diagnosis study in the UK.14 This study also showed that, compared with patients with metastatic cancer of known primary, those with CUP were less likely to have a specialist consultation or an invasive diagnostic procedure, and more likely to have a non-invasive diagnostic procedure.12
The CUP subtypes and observed pattern of diagnostic investigation in elderly Australians suggest under-investigation in some patients with CUP.12 However, this pattern of care is consistent with clinical guidelines, which advocate conservative approaches when the prognosis is poor and the therapeutic plan is palliative rather than curative. Published CUP clinical practice guidelines recommend diagnostic pathways that vary depending on the extent of metastasis, the involved site(s), the suspected origin and the overall health of the patient.3,15 For patients who undergo tumour biopsy or excision, immunohistochemistry can help to identify the subset with a favourable prognosis and guide treatment on the basis of the suspected tumour type. Genomic profiling has the potential to further increase the diagnostic yield by identifying profiles that are consistent with a specific origin or behaviour.16–18 A pragmatic approach is usually taken, which involves targeted diagnostic investigations and treatment of ‘treatable’ malignancies.
Treatment
Patterns-of-care data for Australian DVA clients with CUP indicate that only 30% received cancer treatment,19 which is in close alignment with the experience in the Netherlands20 and Canada.21 CUP tumours generally do not respond to systemic therapies, exhibiting aggressive behaviour and unpredictable metastatic spread. However, locoregional or systemic therapies are recommended in patients with favourable prognostic profiles defined on the basis of clinical and pathological criteria (Table 1).11 In such cases, the behaviour suggests a specific tumour type and treatment algorithms (eg isolated axillary lymphadenopathy, with outcomes usually equally efficacious as those for proven breast cancer). A minority (15–20%) of patients with histopathologically confirmed CUP have a favourable prognosis because their malignancy on immunohistochemistry closely resembles a major tumour. The best example is the presence of hormone receptor positivity, which warrants the use of hormone receptor blockers such as tamoxifen. Increasingly, the use of genomic sequencing will enhance therapeutic options and, thus, outcomes for patients with CUP. However, the diagnostic benefit to date is yet to translate to clinical benefit.22 This situation is expected to evolve rapidly as whole-genome sequencing is performed and actionable mutations are identified.23,24
Table 1. Clinicopathological prognostic subtypes of cancer of unknown primary site (CUP)
|
|
Unfavourable subset
|
Favourable subset
|
|---|
|
Adenocarcinoma metastatic to the liver or other organs
|
Poorly differentiated carcinoma with midline distribution (extragonadal germ-cell syndrome)
|
|
Multiple cerebral metastases
|
Females with papillary adenocarcinoma of peritoneal cavity
|
|
Multiple lung or pleural metastases
|
Females with adenocarcinoma involving only axillary lymph nodes
|
|
Multiple metastatic lytic bone disease (non-prostate-specific antigen [PSA])
|
Squamous cell carcinoma involving cervical lymph nodes
|
|
Squamous-cell carcinoma of the adomino/pelvic cavity
|
Isolated inguinal adenopathy (squamous carcinoma)
|
|
Squamous abdominopelvic CUP
|
Poorly differentiated neuroendocrine carcinoma
|
|
|
Males with blastic bone metastases and elevated PSA (adenocarcinoma)
|
|
|
Adenocarcinoma with a colon-cancer profile (cytokeratin-20+ [CK20+], cytokeratin-7 [CK7-], CDX2+)
|
|
|
Single potentially resectable tumour
|
|
|
Merkel cell adenopathy of unknown origin
|
|
Reproduced with permission from John Wiley and Sons, from Kamposioras K, Pentheroudakis G, Pavlidis N. Exploring the biology of cancer of unknown primary: Breakthroughs and drawbacks. Eur J Clin Invest 2013;43:491–500.
|
Integrated care
Patients with CUP have high care needs, which are often unmet and in excess of those with known primary site.25 Many have a rapid progression to end-of-life care; median survival estimates from population-based studies range from 5–12 weeks.6,19,20,26,27 The median survival for patients with confirmed CUP is 6–7 months for unfavourable subtypes and 12–36 months for favourable subtypes.1
Data from DVA clients show higher rates of general practice consultations, palliative care, hospitalisations and emergency department visits in the 3 months after diagnosis, compared with those with metastatic cancer of known primary site.19 Patients with CUP will benefit from an integrated care plan, regardless of whether they receive curative or palliative, end-of-life care. This care plan must include psychological assessment. Coping with a diagnosis of metastatic cancer is more difficult when the primary site is unknown, and adds to distress, hopelessness, somatisation, anxiety and depression.22 Unsurprisingly, there is also evidence that patients with CUP have poorer health-related quality of life, compared with patients with metastatic and non-metastatic cancer of known primary site.25
Practice implications
CUP is challenging for patients, their families and medical practitioners. Diagnostic difficulties, poor survival rate and absence of recognised specialist clinicians combine to make this cancer very difficult to research outside a cohort setting. As a result, there are numerous evidence gaps and opportunities for patients to slip through the cracks. Although we understand very little about the behavioural risk factors for CUP, general practice interventions that reduce established carcinogenic behaviours such as smoking, excessive alcohol consumption, obesity and sun exposure are expected to reduce the incidence of CUP.
We do not know whether targeted public health campaigns are effective in reducing the presentation time, particularly for patients diagnosed with malignancy of unknown origin, where there is no natural history data. There may be scope for improvements in profiling high-risk individuals presenting to primary care, in whom a workup to rule out malignancy may result in earlier diagnosis. Patients who present with rapid weight loss, especially in the context of pain and/or increasing fatigue and declining activity, should be considered at risk. GPs should perform a thorough history, symptom assessment and physical examination including for lymphadenopathy, liver enlargement, pleural effusion and breast or pelvic abnormality. They may also consider ordering a complete blood count, tumour marker tests (alpha-fetoprotein, protein-specific antigen, cancer antigen-125, human chorionic gonadotrophin) and computed tomography (CT) scans of the chest and abdomen.
The high rates of emergency department visits before and after diagnosis suggest gaps in care, or may simply reflect the need for urgent attention given the aggressive nature of CUP. We lack evidence on the patterns of care associated with improved outcomes for this patient group.
Although most patients with CUP have poor prognoses, a recognised subset has a favourable prognosis and must be offered treatment according to published guidelines. Early identification of these patients will maximise their response to therapy. Multi-agent cytotoxic therapies are usually reserved for those with good functional status. Patients with CUP will benefit from comprehensive, coordinated care that is patient-centred and acknowledges their unique vulnerability and healthcare needs. This includes avoiding investigations and treatments that will not improve prognosis, and focusing on those that address symptoms and minimise the risk of hospitalisation. GPs have an important role in ensuring continuity of care, communication and collaboration between specialists, and communication with the patient and family. This would be aided by Australian guidelines and policies for the follow-up and care of patients with CUP.
Authors
Claire M Vajdic BOptom (Hons), PhD, Head, Cancer Epidemiology Unit, Centre for Big Data Research in Health, University of New South Wales, Sydney, NSW. claire.vajdic@unsw.edu.au
David Goldstein MBBS, FRACP, Senior Staff Specialist, Department of Medical Oncology, Prince of Wales Hospital, University of New South Wales, Sydney, NSW
Competing interests: David Goldstein’s institution has received grants from Celgene, Amgen and Pfizer.
Provenance and peer review: Commissioned, externally peer reviewed.