Prostate cancer affects one in five men and accounts for over 3000 deaths in Australia each year.1,2 Most men present with disease that is confined to the prostate (localised prostate cancer).3 On the basis of prostate-specific antigen (PSA) levels, digital rectal examination findings and Gleason score at diagnosis, localised prostate cancer can be stratified into low-risk intermediate-risk and high-risk groups.4 Individual treatment recommendations are best made within the context of a multidisciplinary team,5 and should reflect risk groups (Table 1) in combination with patient age, baseline symptoms, medical comorbidities and preferences. A very low-risk group is also recognised (≤cT1c, Gleason score ≤6, PSA <10 ng/mL, fewer than three positive prostate biopsy cores, ≤50% cancer in any core, PSA density <0.15 ng/mL/g) in whom active surveillance may be appropriate.6 This is an important consideration as PSA screening becomes more widely utilised.
Table 1. Treatment options for localised prostate cancer according to risk group
|
|
|
Low risk
|
Intermediate risk
|
High risk
|
|---|
|
PSA
|
0–10 ng/mL
|
10–20 ng/mL
|
20 ng/mL or higher
|
|
Clinical T-stage
|
T1–T2a
|
T2b
|
T2c–T4
|
|
Gleason score
|
6 or lower
|
7
|
8 or higher
|
|
Management options
|
- Active surveillance
- RP
- EBRT
- LDR BT
|
- RP
- EBRT +/– ADT
- EBRT + HDR BT +/– ADT
|
- EBRT + ADT
- RP +/– adjuvant/salvage EBRT +/– ADT
- EBRT + HDR BT + ADT
|
|
Low-risk, intermediate-risk and high-risk groups are defined according to D’Amico classification3
PSA, prostate specific antigen; RP, radical prostatectomy; EBRT, external beam radiotherapy; ADT, androgen deprivation therapy; BT, brachytherapy; LDR, low dose rate; HDR, high dose rate; T1c, tumour identified by needle biopsy, clinically palpable; T2a, tumour involves half of one lobe or less; T2b, tumour involves more than half of one lobe but not both lobes; T2c, tumour involves both lobes; T4, tumour is fixed or invades adjacent structures other than seminal vesicles (eg external sphincter, rectum, bladder, levator muscles, and/or pelvic wall)
|
External beam radiation therapy (EBRT) is an established treatment for prostate cancer, but technological advances over the past decade have changed the way it is delivered. This article will review these developments and their impact on patient-relevant outcomes. The authors’ objective is to assist general practitioners (GPs) in discussing EBRT for localised prostate cancer with patients.
Modern EBRT for prostate cancer and patient experience
Most Australian men with localised prostate cancer treated with curative intent using EBRT receive conventionally fractionated, dose-escalated treatment. This treatment consists of 1.8–2 Gy per fraction given every day, five times per week, for a total dose of at least 74 Gy in 37 fractions over 7.5 weeks.7 Doses above 74 Gy decrease the risk of biochemical failure and improve prostate cancer-specific survival, but not overall survival.8 EBRT is delivered with a linear accelerator (linac), which generates megavoltage photon beams that can be oriented and shaped to match a patient’s unique tumour position, size and shape. A photograph of a modern linac is shown in Figure 1.
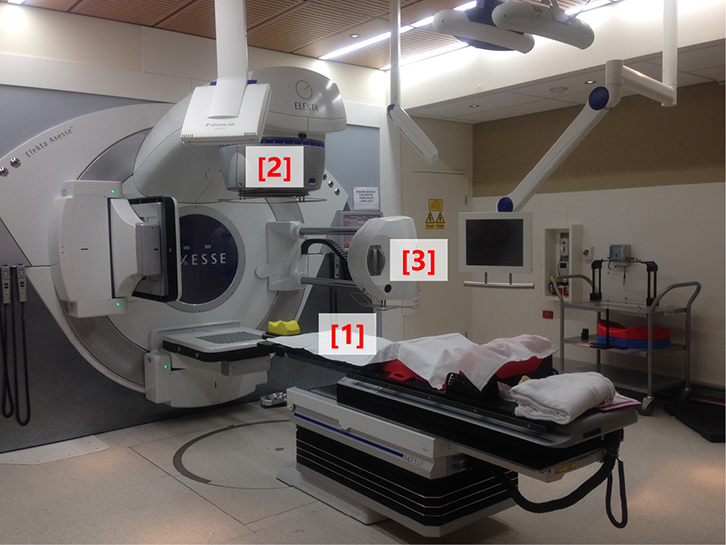 |
Figure 1. Photograph of a modern linear accelerator
The patient lies supine on the treatment couch (1); the radiotherapy beam exits the treatment head (2), which is mounted on a gantry that can rotate around the patient to deliver beams from different positions;
on-board cone beam CT equipment (3) is available for image guidance |
Fiducial seeds, if used, are inserted a few weeks before radiotherapy planning. The process and experience are similar to those of prostate biopsy. The patient typically lies supine for EBRT on a hard treatment table. Minimal cushioning is used to reduce variation in body position. The table is moved into position according to a laser-coordinate system. Image guidance may be performed just prior to treatment to ensure optimal target localisation. Each fraction takes 2–10 minutes, depending on body habitus and EBRT technique. Treatment is delivered as an outpatient procedure, and the patient is monitored regularly by a team including a radiation oncologist, specialist nurses and radiation therapists. After treatment, the patient may continue follow-up with his radiation oncologist and/or urologist. The treatment response is assessed primarily according to PSA, which can take months or years to reach a nadir after EBRT.
Androgen deprivation therapy (ADT) may also be given before, during and/or after EBRT, to cytoreduce and radiosensitise the prostate cancer, depending on the risk profile. The use of ADT may be associated with separate side effects and can confound PSA interpretation through direct effects that are independent of EBRT.9
Expected side effects with modern EBRT
Irritative and/or obstructive lower urinary tract symptoms caused by radiation-induced cystitis and urethritis are common during radiotherapy. Generalised fatigue and rectal symptoms (eg urgency and tenesmus) may also be seen. Most acute toxicities develop gradually from weeks 3–4 of treatment. Commonly, these toxicities are mild or moderate in severity, can be managed with simple treatments and resolve within 4–6 weeks after treatment concludes.10
Following completion of EBRT, late toxicities relating to fibrotic and/or vascular changes may manifest months or years after treatment. This affects <10% of men.11 In most cases, they will settle without intervention, but should be investigated appropriately to exclude other causes.
Moderate severity (grade ≥2) late urinary toxicity (eg urgency, dysuria and haematuria) and late rectal toxicity (eg altered bowel habit, urgency and rectal bleeding) is expected in up to about 5% of men 3 years after treatment.12 The incidence of persistent, long-term complaints does not differ greatly from those reported in the general population.13 However, they are more common in patients with significant pre-treatment urinary and bowel symptoms, a history of abdominal surgery and anti-coagulant use. Erectile dysfunction does occur more commonly after EBRT over time, but not immediately, as is the concern following radical prostatectomy. Erectile dysfunction is related to increasing age, pre-treatment erectile function and use of ADT. Men with no contraindications to phosphodiesterase type 5 inhibitors can be trialled on these medications. The risk of radiotherapy-induced second malignancy many years later is small14 and is most relevant to those with many decades of expected survival.
Comparison of efficacy and side effects between EBRT and surgery
EBRT and radical prostatectomy are the two most commonly used treatment modalities, but have never been compared in a randomised trial. Non-randomised data are prone to selection bias, but suggest equivalence in terms of prostate cancer control15–17 in the majority of cases. Differences in risk and side effect profiles between the two modalities (Table 2), and their relative importance attributed by an individual patient are likely to influence treatment selection.
Table 2. Comparison of radical prostatectomy and radiotherapy
|
|
Radical prostatectomy
|
External beam radiation therapy
|
|---|
- In-patient stay usually fewer than 7 days, followed by 4-week recovery at home
- Potential complications:
- anaesthetic and peri-operative risk
- urinary incontinence
- vesico-ureteric (bladder neck) stricture
- early erectile dysfunction
- rectal injury
- infertility
- Adjuvant external beam radiation therapy may be recommended for high-risk pathological features (involved margins, seminal vesicle involvement, extra-capsular extension)
|
- Outpatient treatment over 7–8 weeks
- Must be able to lie flat for 30 minutes
- Potential complications include urinary symptoms, bowel symptoms, late erectile dysfunction, second malignancy (very rare), infertility
- Side effects of neo-adjuvant androgen deprivation therapy (if used in combination with radiation therapy) include hot flushes, fatigue, loss of libido, mood disturbance, weight gain, bone demineralisation
|
The Prostate Cancer Outcomes Study evaluated 2365 American men with localised prostate cancer who were treated in 1994–95, with at least 24 months follow-up.18 Rates of satisfaction were high for patients undergoing either treatment. However, differences in outcomes were found, including:
- urinary leakage (daily or more often in 12% EBRT versus 35% radical prostatectomy)
- bowel urgency (almost every day in 3% EBRT versus 1% radical prostatectomy)
- complete erectile dysfunction (43% EBRT versus 58% radical prostatectomy in contrast to 86% receiving ADT monotherapy).
Evolution of radiotherapy technology
Historically, accurate localisation of the prostate for EBRT was difficult because it is a mobile structure and is not visible on plain radiographs. Consequently, EBRT fields were defined according to bony landmarks used as surrogates for prostate position. The dose delivered to the tumour was limited by the tolerance of the normal tissues (eg rectum and bladder) that were also exposed within these large fields.
Computed tomography (CT)-based EBRT planning was introduced in the 1980–1990s, and heralded the 3D-conformal radiotherapy (3DCRT) era. CT planning allows the radiation oncologist to delineate the anatomical extent of the prostate and nearby organs at risk in three dimensions on axial images. It also enables multiple-shaped beams to be oriented and shaped around the target, to reduce high doses to organs at risk. This enabled 3DCRT to deliver a higher radiation dose to the prostate, improving prostate cancer control rates and reducing treatment-related side effects.19 In the past 20 years, further technological advances have improved our control over where the high radiotherapy doses are deposited within the patient, target volume delineation and methods to account for prostate motion.
Intensity-modulated radiation therapy
Intensity-modulated radiation therapy (IMRT) is an advanced form of 3DCRT. Multi-leaf collimators (Figure 2) are used to vary the number of photons (intensity) across the radiotherapy beam, creating dose distributions that tightly conform to the target volume and further spare dose to organs at risk. Standard IMRT is delivered using multiple static fields. Figure 3 illustrates how IMRT can sculpt doses around target volumes and away from organs at risk.
 |
Figure 2. Multi-leaf collimators
These are motorised tungsten leaves located within the treatment head used to deliver conformal radiotherapy |
|
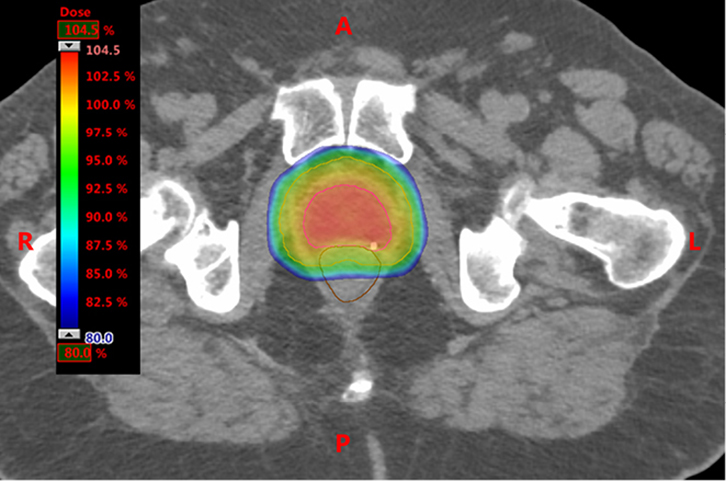 |
Figure 3. Axial slice through planning CT scan
The dose colour wash shows dose distributions achievable with intensity-modulated adiotherapy
Note the homogenous coverage of the planning target volume (turquoise line) and sparing the rectum (brown line) |
|
Rotational IMRT
Rotational IMRT is a sophisticated technique that delivers highly conformal treatment from an infinite number of beam angles using a rotating linac head. Rotational IMRT is not expected to be superior to standard IMRT in terms of prostate cancer control, but is widely used across Australia because it can deliver each fraction in a shorter time (2–5 minutes). This increases department capacity, is more convenient for the patient, and reduces the risk of tumour and organs at risk motion during treatment. Machines used to deliver rotational IMRT include VMAT (Elekta, Stockholm, Sweden), RapidArc (Varian Medical Systems, Palo Alto, CA, USA) and TomoTherapy (Accuray, Sunnyvale, CA, USA).
IMRT radiotherapy planning
A dedicated CT scan of the pelvis is required to plan EBRT for localised prostate cancer. The radiation oncologist uses the CT scan to accurately delineate the target volume (including whole prostate) and organs at risk (eg rectum, bladder and femoral heads), then prescribe an appropriate radiation dose and organs at risk dose constraints. Some patients may also undergo magnetic resonance imaging (MRI) of the pelvis, which can be fused with the planning CT to aid in target volume delineation, given its superior soft tissue differentiation.
The final target volume is methodically constructed to account for microscopic tumour spread, physiological movement, and other mechanical and physical uncertainties inherent to the treatment process. Radiation therapists use sophisticated dose-calculation algorithms to create a radiotherapy plan based on the individual patient’s internal anatomy. A quality assurance process takes place once the plan is reviewed and approved by the radiation oncologist, to ensure it can be delivered reliably. The whole process usually takes about 2 weeks.
Accounting for organ motion
The prostate is a mobile organ with near-continuous motion influenced by bladder and rectal filling. The position of these structures as defined on the planning CT can vary during and between fractions. Delivery of highly conformal treatments with steep dose gradients demands confidence in localisation of the target because motion can lead to geographic miss, under-dosing of the tumour and/or over-dosing of organs at risk. Methods to account for organ motion are described under the following four sections.
Minimising daily variability – bladder and bowel protocols
Patients may be asked to regulate their bowel habits during the planning and treatment period through dietary modification and/or medication. Patients repeat the same routine before each fraction is delivered (eg holding a comfortably full bladder and maintaining an empty rectum).
Visualising motion – fiducial seeds
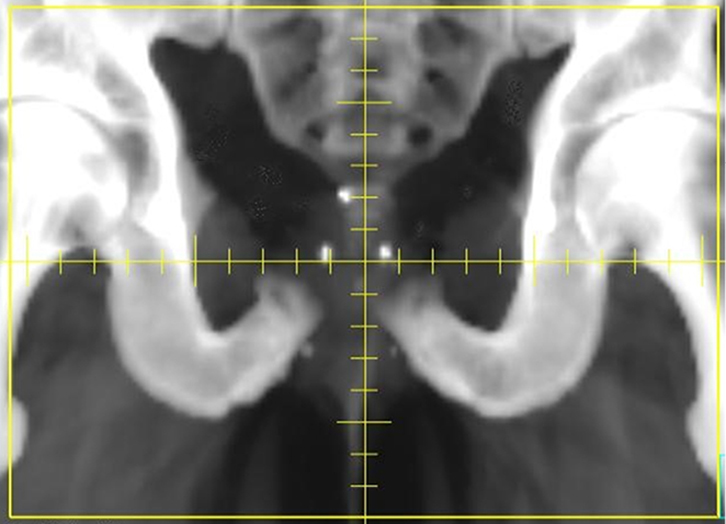 |
| Figure 4. Digitally reconstructed radiograph highlighting the position of three radio-opaque gold seed fiducials |
Inert, radiopaque fiducial seeds may be permanently inserted into the prostate under transrectal or transperineal ultrasound guidance. The seeds can be used as a surrogate for prostate position, and displacement can be estimated before each fraction, using orthogonal 2D radiographs (Figure 4). The radiation required to take these images can be factored into the overall treatment dose. Shifts in the treatment table can be made to re-position the patient and seeds (and therefore prostate) according to the location on the planning CT.
Visualising motion – on board cone-beam CT (CBCT)
Dedicated CBCT equipment can acquire a 3D CT image in real-time in the treatment position just before treatment. Resolution is not of diagnostic quality but enables visualisation of soft tissues (prostate, bladder and rectum) so that table shifts can be made if needed. CBCT can be used in conjunction with fiducial seeds.
Visualising motion – real-time motion tracking systems
Systems including Calypso (Varian Medical Systems, Palo Alto, CA, USA) use radiofrequency transponders inserted directly into the prostate to monitor motion. Currently, such systems are not widely used in Australia.
Future directions: hypofractionated radiotherapy for prostate cancer
Hypofractionated EBRT delivers equivalent or greater total doses in a shorter overall treatment time than conventional fractionation, which delivers higher doses per fraction. It is attractive because prostate cancer may be more sensitive to hypofractionation and it allows patients to complete treatment more quickly. Data from randomised trials assessing this approach for localised disease are awaited; at short follow-up, however, it appears to be well tolerated.20
Stereotactic body radiotherapy (SBRT) is a form of extreme hypofractionation where the entire course is completed in about five fractions over a week. It builds on technological advances in image guidance and robust quality assurance developed initially for use in intracranial stereotactic radiosurgery. SBRT technology is available in Australia, but its use in the treatment of localised prostate cancer remains investigational. Patients may wish to consider enrolment in clinical trials evaluating SBRT efficacy and toxicity.
ADT and other systemic therapies are the primary treatment for most patients with metastatic disease. However, a subset of men with a more favourable prognosis may benefit from local therapies. SBRT for oligometastatic disease could maximise durable local control of bony metastases, prevent symptomatic progression and delay the need for systemic therapies in those with more indolent disease. Further evaluation is ongoing and considerable radiation oncologist expertise is needed to ensure optimal patient selection and safety, given the very high doses and steep dose gradients required.
Conclusion
EBRT for prostate cancer has changed dramatically over the past two decades, resulting in improvements in disease control and treatment-related toxicity. Image-guided IMRT is now the standard of care across Australia, creating highly conformal treatments that maximise the dose delivered to the target while sparing normal tissues. The majority of men experience mild, acute side effects during treatment, but <10% experience significant late urinary or bowel symptoms. Recent advances in technology are being evaluated in select groups of men with localised or metastatic disease that may further improve outcomes and reduce treatment times in the future.
Authors
Peter Gorayski BSc (Hons), BMBS, FRACGP, FRANZCR, Radiation Oncology Queensland, Toowoomba, QLD; School of Medicine, University of Queensland, Brisbane, QLD. peter.gorayski@gmail.com
Mark B Pinkham BMBCh, MA (Hons) (Oxon), FRANZCR, Princess Alexandra Hospital, Woolloongabba, QLD; School of Medicine, University of Queensland, Brisbane, QLD
Margot Lehman MBBS (Hons) FRANZCR GDPH, Princess Alexandra Hospital, Woolloongabba, QLD; School of Medicine, University of Queensland, Brisbane, QLD
Competing interests: None.
Provenance and peer review: Not commissioned, externally peer reviewed.