New mothers frequently experience breastfeeding problems and many seek support.1,2 In the first year, mothers and infants make an average of 36 visits to healthcare providers, eight of those to their general practitioner (GP).3 Healthcare providers approached by new mothers must be able to adequately assess breastfeeding problems and provide solutions. Women find it unhelpful when health professionals suggest they stop breastfeeding. Incorrect advice is a major cause of premature cessation of breastfeeding.4-6
Challenges to breastfeeding are common and persistent; our recent study of 340 primiparous women in Melbourne found that 79% experienced nipple pain and 58% nipple damage.7 Although many women continue to breastfeed despite experiencing considerable pain, nipple pain is the second most common reason given for women ceasing to breastfeed before they had planned to do so, the first being perceived low supply.8
Nipple pain is generally attributed to mechanical stressors, physiological responses and infection.9 Poor latch is the most common cause of damage to nipples.9 Any nipple distortion or bruising of the skin is indicative of a significant mechanical problem. About 50% of breastfeeding women complain of damage to their nipples, which increases the risk of skin infections and mastitis.9 The assumption is that damage to nipple tissue causes pain and that pain is an indicator of tissue damage. Considering the extensive literature on pain and neuroplasticity, including central reorganisation, the relationship between tissue pathology and pain is more sophisticated than this.10 In contrast to vulval pain, where the possible interacting roles of candidiasis, dermatitis and allodynia are acknowledged,11 we believe the understanding of nipple pain in lactation has been overly simplistic.
To enhance current practice, we have proposed an integrated approach to the assessment of breastfeeding pain. Incorporating current concepts from neuroscience relating to neuroplasticity12 and pain,13 we use a biopsychosocial lens to review possible determinants of the nipple pain experience. We adapted a template originally designed to assist clinical reasoning for musculoskeletal pain,14 to help the clinician to recognise the potential contributors to women’s nipple pain, and to categorise these in terms of pain mechanisms. This enables the identification of treatment targets on the basis of the predominant contributors. We provide brief case studies to illustrate the increased management options that become available when a comprehensive range of contributors is considered.
The template
The template refers to the ‘Pain and Movement Reasoning Model’, a reasoning tool developed to incorporate the range of influences and determinants of pain identified in current literature.14 This model is represented by a gridded triangle. The apices refer to three categories: ‘local stimulation’, ‘regional influences’ and ‘central modulation’. These categories are interdependent (eg regional influences may lead to local stimulation mechanisms). After considering the clinical information relevant to the three categories, the clinician marks a point on the grid to best represent clinical judgement of the relative contribution of each. This ensures the consideration of the range of potential contributors to pain and results in holistic interpretation of a person’s pain report. This clinical reasoning tool therefore enables the clinician to consider the multiple dimensions of pain in the decision-making process.
We have modified the Pain and Movement Reasoning Model template to address nipple pain. Our version is called the Breastfeeding Pain Reasoning Model (Figure 1). The category headings were slightly modified to best represent the breastfeeding context: local stimulation, external influences and central modulation. These categories are described below.
Local stimulation
In the Breastfeeding Pain Reasoning Model, ‘local stimulation’ refers to mechanical stimuli, such as nipple compression, but also encompasses inflammatory and infective states that contribute to nociception. Mechanonociceptors respond to strong mechanical stimuli that cause distortion of the nipple tissue. Where these stimuli are of sufficient strength and frequency to cause breakdown of the skin, cytokines, such as gamma-interferon, interleukins 1 and 6, and tumour necrosis factor are released.13,15 As a result, chemonociceptors are activated and the inflammatory response is initiated with contribution from afferent antidromic release of substance P and calcitonin gene related peptide (CGRP), which are both vasodilators.15 The inflammatory exudate has been labelled a ‘sensitising soup’ and contains substances that have the ability to further activate and sensitise nociceptors – peripheral sensitisation.16 These substances include noradrenaline, bradykinin, histamine, prostaglandins, serotonin, nerve growth factor and cytokines.15,16 Nociceptors in cracked and damaged nipple tissue will become sensitised, leading to activation of mechanical nociceptors and other afferents at reduced thresholds. Damaged nipple skin is likely to be colonised with bacteria (eg Staphylococcus aureus) and/or fungi (eg Candida albicans).9 Recent evidence suggests that pathogenic bacteria not only trigger an inflammatory response, but can activate nociceptors directly.17 Importantly, the neural transmission from an activated nociceptor is not pain, but deactivating the nociceptor or removing the threatening stimulus may reduce or relieve pain. This would especially be the case in less complex presentations, where central nervous system (CNS) factors have only a small role in the woman’s pain experience.
External influences
The external influencing category includes factors that might contribute to the woman’s nipple pain experience but are not due to a pathological process. That is, they are not likely to provoke an inflammatory response but may predispose, exacerbate or contribute to the process. In the context of nipple pain, we have divided this category into four subcategories: attributes of the mother, attributes of the infant, the interaction between mother and infant, and finally of miscellaneous other external influences (eg breast pump).
Attributes of the mother that might contribute to the woman’s experience of nipple pain include the shape of nipple and its adaptability to any distortion. During breastfeeding, the nipple needs to be sufficiently elastic to be positioned deep in the infant’s mouth, which may not be possible for some women with flat or very short or wide nipples.18
Attributes of the baby contributing to maternal pain include anatomy of the mouth, such as size and shape of mouth, palate and tongue. Babies with very small mouths, receding chins, high palate or restricted tongue movements may be hard to latch without maternal pain. Restriction of tongue movement may be obvious in some infants with classic tongue-ties (ankyloglossia),19 but less obvious restriction occurs in some infants with posterior tongue-ties.20
The third subcategory relates to the interaction between infant’s mouth and mother’s nipple and breast. When the infant is well positioned on the breast, the nipple is deep in the baby’s mouth and movement of the tongue and jaw is felt as a drawing sensation by the mother. In this situation, the nipple is not distorted after the feed and the nipple skin is undamaged. Poor attachment or latching can lead to visible compression of the nipple after the feed, and may lead to skin breakdown.21
The last subcategory refers to other external contacts with the nipple: topical products (eg soap and creams), products such as pads (breast pads for absorbing milk leakage, or hydrogel or other dressings) and breast pumps. Some products used on the nipple and areola may cause an irritant or allergic dermatitis.22 An important external agent is the breast pump, which most new mothers are using.23 Breast pumps can cause nipple damage if the flange is too tight, the suction too high or the pump used for too long.24
Central modulation
Input from the peripheral nervous system can be amplified or inhibited via CNS processes. Amplification of neural activity or loss of normal inhibition can result in a sensitised state. There is a vast array of factors that alter CNS sensitivity. First, a prolonged inundation of impulses through sensory fibres can enhance neural transmission at the spinal cord.10 Changes that can occur at the dorsal horn of the spinal cord include increased neurotransmitter production presynaptically and the production or relocation of more receptors postsynaptically. Neuroplastic alterations to receptors also make them more easily activated, increasing the transmission to second-order neurons and higher centres. In practice, this may translate to the woman having an increased pain response to tissue deformation in the affected breast.
Genetic predisposition and expression of phenotypes related to pain sensitivity also need to be considered and perhaps explains the comorbidity of some pain conditions.25 Glial cells and the immune system have been identified as having a major influence on pain.26 Arguably, pain is just the most overt sign of the body’s protection system. Therefore a woman who is unwell, particularly with infective or inflammatory conditions, may be predisposed to be more sensitive to other noxious stimuli.
Importantly, factors that reduce the inhibition initiated by higher centres can also cause more excitation in these second-order neurons.13 This disinhibition leads to a state of increased sensitivity and is influenced by factors such as fatigue, lack of social support, sense of failure, beliefs about the risk of harm, anxiety and low mood. Notably, sleep deprivation increases sensitivity to pain (hyperalgesia), especially hyperalgesia to cold,27 so it is not surprising that new mothers who experience weeks or months of interrupted sleep may complain of increased sensitivity to cold environments. Nipple pain associated with nipple vasospasm has been reported anecdotally in women predisposed to poor circulation or Raynaud’s phenomenon.9 Social distress associated with loneliness or feelings of rejection is linked to higher pro-inflammatory activity and sensitivity to pain.28 Holding the hand of a loved one can reduce measurable pain-related neural activity as well as self-reported pain.28
Management options
The Breastfeeding Pain Reasoning Model gives us a structure to consider categories of management: local, external and central. Table 1 provides examples of management options. Local stimulation can be reduced by treatments that improve healing, such as purified lanolin, or application of topical antibiotics or corticosteroids, as appropriate. Reducing external stimulation can include improving attachment of the infant to the breast, or improving the use of the breast pump (ensuring flange is not too tight, using lubricant – lanolin or sunflower oil – before pumping), avoiding excessive time of either baby or pump on the breast, removing/reducing irritants – creams, breast pads – avoiding cold, applying hot packs or ‘breast warmers’. Other strategies, such as the use of a nipple shield or dummy, or expressing and bottle/cup feeding to reduce duration of nipple trauma, may be appropriate. Central modulation can be managed by strategies such as increasing maternal rest, massage for the upper thoracic/neck region, and social support, as well as centrally acting medications that alter nerve function. Understandably, some women who experience nipple pain think the worst of the situation, with magnification of pain symptoms, rumination and feelings of helplessness and pessimism.29 Psychological techniques such as distraction, cognitive reframing, relaxation and fostering effective coping strategies may be beneficial.30
Table 1. Management options using the breastfeeding pain reasoning model
|
|
Categories
|
Examples of management strategies
|
|---|
|
Local stimulation
|
Improve healing:
- wash nipple regularly
- use antibacterial pads or hydrogel dressings
- apply purified lanolin before and after feeding/expressing
Localised infection:
- apply topical antibiotic ointment (eg mupirocin 3 x/day after feeds) or antifungal (eg miconazole oral gel 4 x/day after feeds)
Local inflammation:
- apply topical steroid (eg mometasone ointment once/day after feeds)
|
|
External influences
|
Improve attachment of baby to breast33
Reduce sources of nipple trauma:
- release infant tongue-tie (if present)
- user lower setting on breast pump
- hire more effective breast pump
Reduce friction from breast pump:
- apply lubricant (sunflower oil, lanolin) prior to expressing
- ensure correct size flange is being used
Trial of nipple shield
Remove sources of irritation:
- creams/gels
- avoid soap, chlorine swimming pools, other irritants
Temporarily reduce duration/frequency of feeds/expressing (supplementation with infant formula may be required if milk supply not sufficient)*
|
|
Central modulation
|
Manage pain elsewhere (Table 2):
Improve maternal rest and sleep:
- arrange child care or reduce mother’s home duties
Maximise comfortable positions for feeding:
- try laid-back feeding or reclining as on a deck chair rather than sitting upright or hunching forward
If cold is a factor:
- avoid airing nipples
- keep nipples warm
- apply heat pack
- try magnesium supplement
- consider nifedipine (commence with 20 mg sustained release daily and increase PRN)33
Refer for psychological support (Table 2)
Refer to peer support (Table 2)
- ABA, PANDA, new mothers’group or online parenting groups
|
|
*This option is not ideal but may appeal to mothers who are considering stopping breastfeeding completely
|
Application of the Breastfeeding Pain Reasoning Model
 |
 |
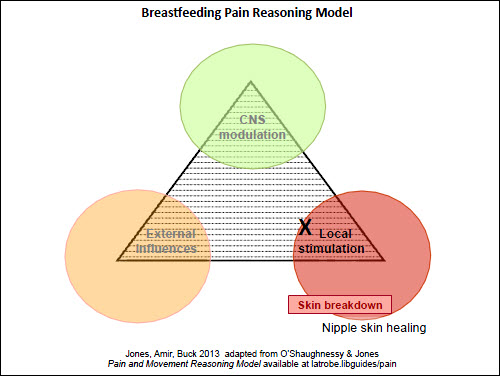
| Figure 2. Pain reasoning model – Case 1 – nipple pain |
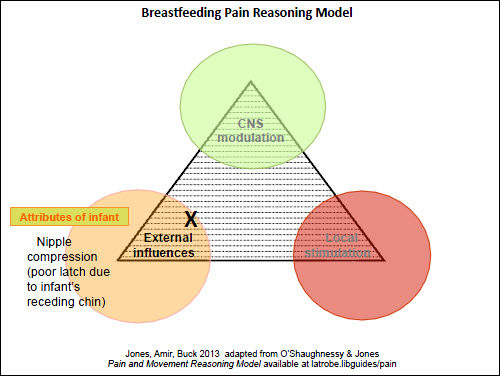
Figure 3. Pain reasoning model – Case 2 – nipple pain |
 |
 |
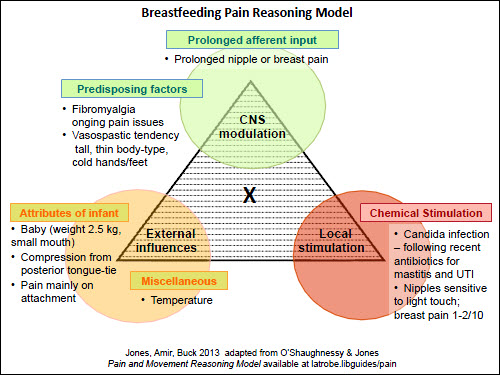
| Figure 4. Pain reasoning model – Case 3 – nipple and breast pain |
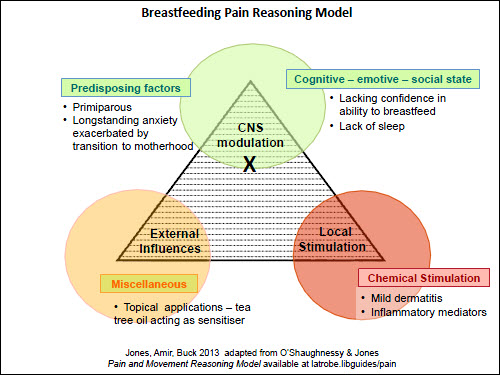
Figure 5. Pain reasoning model – Case 4 – nipple and breast pain |
Keeping the Breastfeeding Pain Reasoning Model in mind, the clinician can consider the various inputs into the mother’s pain experience and the relative importance of local, external and central factors (Figure 1). The clinician can plot their assessment of the pain experience onto the grid, or just keep it in mind, as they move on to explain the situation to the patient and recommend treatment options.
The first case study is a straightforward situation of a mother at day 5 postpartum, who has experienced superficial skin damage due to poor positioning of her infant on the breast initially, but now has nipple skin healing and minimal pain (local factors: Figure 2). A scenario where the nipple pain occurs solely when feeding the baby directly at the breast, with no pain when expressing or between feeds, can be seen in Figure 3 (external factors). It could be argued that there is less need for the Model in these simpler presentations; however, it is important that the clinician is alert to factors that may lead to persistent nipple pain and addresses them early.
The usefulness of the model is more apparent when women present with ongoing nipple pain. Case 3 is a more complicated scenario: the mother has a history of fibromyalgia and, in the past, had recurrent vaginal thrush following antibiotics (Figure 4). Her baby weighed 2.5 kg at birth and had a small mouth, causing some mechanical damage to her nipples. The mother required antibiotics for the first 2 weeks postpartum for a urinary tract infection and for mastitis. At 4 weeks postpartum, she had burning, sensitive nipples and some radiating breast pain. On the commonly used pain score, where 0 equals no pain and 10 is the worst pain possible, she reported the nipple sensitivity of 1–2 out of 10. In this scenario, the clinician can explain to the mother that there are a number of factors contributing to her pain: local infection (nipple/breast candidiasis is causing the low level nipple sensitivity), external trauma (baby’s small mouth is causing the pain on attachment) and central modulation (caused by her co-existing chronic pain condition). By acknowledging the contributing factors, the focus is not reliant on multiple courses of antifungals to achieve pain-free breastfeeding, and other options can also be suggested (Table 1). Using pain scores helps us to estimate the input of each of these pain sources.
The last scenario, shown in Figure 5, is of a woman with a long history of anxiety (Case 4). Her anxiety about nipple sensitivity led her to apply tea tree oil as an antifungal treatment/preventive measure. However, tea tree oil is a common skin irritant31 and caused a mild dermatitis. Considering each apex of the triangle provides opportunities for a range of management and referral options. Table 2 provides the GP with a wide range of possible sources of support for the patient with ongoing breastfeeding pain.
Table 2. Referral options
|
|
Categories
|
Examples
|
|---|
|
Breastfeeding support
|
International Board Certified Lactation Consultant*
Hospital breastfeeding service
Community breastfeeding clinic
Australian Breastfeeding Association (ABA)†
|
|
Psychological support
|
Clinical psychologist
General practitioner
Psychiatrist
Relationship counselling
Post and Antenatal Support Association (PANDA)‡
Family/mother–baby units
|
|
Physical support
|
Physiotherapist
Massage therapist
Osteopath
Occupational therapist
|
|
Medical specialist
|
Dermatologist
Musculoskeletal specialist
|
|
Pain management
|
Acupuncture
Hypnotherapy
Pain clinic
|
|
*Lactation Consultants of Australia and New Zealand, www.lcanz.org/find-a-consultant.htm
†www.breastfeeding.asn.au/
‡www.panda.org.au/
|
Conclusion
When the clinician understands the meaning of nipple pain to the woman, and makes use of the range of management options available, there is rarely a need to suggest ceasing breastfeeding, unless that is the mother’s decision.31 By using this Breastfeeding Pain Reasoning Model, clinicians can identify the predominant influences on nipple pain. This enables them to provide realistic advice to women and ensure systems are in place to provide effective support for breastfeeding from appropriate health professionals and community resources (Table 2).32
Key points
- Nipple pain is a common problem for breastfeeding women. Ongoing pain may be multifactorial.
- Our model considers local, external and central factors that contribute to the new mother’s pain experience.
- GPs can use this model to explain the situation to the patient and increase the range of potential management strategies.
Competing interests: None.
Provenance and peer review: Not commissioned, externally peer reviewed.
Acknowledgments
Des O’Shaughnessy (co-author Pain and Movement Reasoning Model) provided the initial framework to enable the comprehensive consideration of neurophysiology of pain for this article.