Burns are a common trauma that affects up to 1% of the Australian population and may be associated with significant physical, psychological, social and economic burden.1 Chemical burns represents 3–5% of all burns-associated admissions.2 Despite the small proportion, chemical burns account for 30% of burns-associated death,3,4 most commonly occurring as a result of chemical ingestion. Given the nature of injury, hospitalisation tends to be prolonged and healing is delayed.
Many substances that are freely available in the community, either occupational or domestic items, have the potential to cause chemical burns. The immediate availability and poor labelling of these substances has accounted for an increase in unintentional chemical burns. Assault and suicidal attempts account for the remaining cases of chemical burns. The affected population is generally evenly distributed but an increase in paediatric chemical burns has been previously documented.5 Areas affected tend to include the face, eyes and extremities. As such, the scope of this review is limited to the assessment and management of cutaneous chemical burns. Ocular burns should be urgently referred to an appropriate ophthalmic service.
More than 25,000 chemicals are used commonly in industrial and domestic settings. The diversity of harmful chemicals results in a vast array of clinical sequelae and a short review would not suitably cover the relevant treatments. The current publication is aimed to provide principals in the assessment and general management of chemical burns.
Pathophysiology and types of chemicals
The pathological end result of chemical burns, regardless of the type of chemical, is consistent with changes occurring during thermal burns. The external toxic stimulus causes denaturation of biological proteins and thus renders them physiologically inactive. This inactivation of essential proteins results in cell death. Thermal burns tend to cause rapid coagulation of protein due to protein crosslinking. By contrast, chemical burns cause denaturation of physiological proteins through six different processes including reduction, oxidation, corrosion, vesication, dessication and protoplasmic poisoning.6,7 It should be noted that many chemicals cause injury through combinations of these processes.
 |
| Figure 1. Severe liquefactive necrosis secondary to exposure of alkaline chemical |
Chemical agents can also be classified on the basis of the induced chemical reaction that the agent initiates. Such classification may be useful for consideration of early management options. Chemical agents may be classified into one of these categories despite slight variations in the resulting clinical sequelae.
- Acids: act as proton donors in the biological system. Acid injury causes a coagulative necrosis of the superficial tissue.
- Bases: chemicals are proton acceptors and tend to have greater capability of producing injury.7,8 These agents produce heat via reactions with fats, extract water from surrounding tissue and result in liquefactive necrosis (Figure 1). Such necrosis allows penetration deep to the superficial wound and continues to cause injury despite initial removal of the insult.9
- Organic solutions: cause injury by dissolving the lipid membrane, which results in disruption of physiological processes.
- Inorganic solutions: cause injury by denaturation mechanisms as outlined above.
Assessment
- Personal protection equipment: it is vital that the treating clinician wears protective clothing to prevent injury (eg gloves, safety googles).
- Primary and secondary survey: as with any clinical presentation, the patient must be stabilised using principals of primary survey. This should be completed in a rapid and systematic approach.
- Airways: ingestion of chemicals, particularly alkali agents, may result in upper airways obstruction. Stabilisation of airways and urgent medical support is required.
- Breathing: special considerations during chemical injury include the exclusion of inhalation injuries, particularly for aerosol chemicals or smoke.10 Such patients frequently require ventilatory support and thus early referral should be sought prior to clinical deterioration.
- Circulation: smaller chemical burns infrequently cause cardiovascular collapse. Occasionally, severe metabolic disturbances may result from chemical absorption and thus monitoring and stabilisation may be required.
- History: a rapid history should be taken simultaneously during primary survey and initial care of the cutaneous burn. It is vital that such assessment does not delay the initiation of immediate treatment. Information regarding comorbidities and medication may be useful. Information regarding the chemical injury is important, particularly if the patient requires transfer to a higher acuity service. Pertinent information includes: insulting agent (and the associated mechanism of injury), phase of the chemical (gas, liquid or solid), concentration, quantity, duration of cutaneous contact, extent of penetration and initial emergency management.7
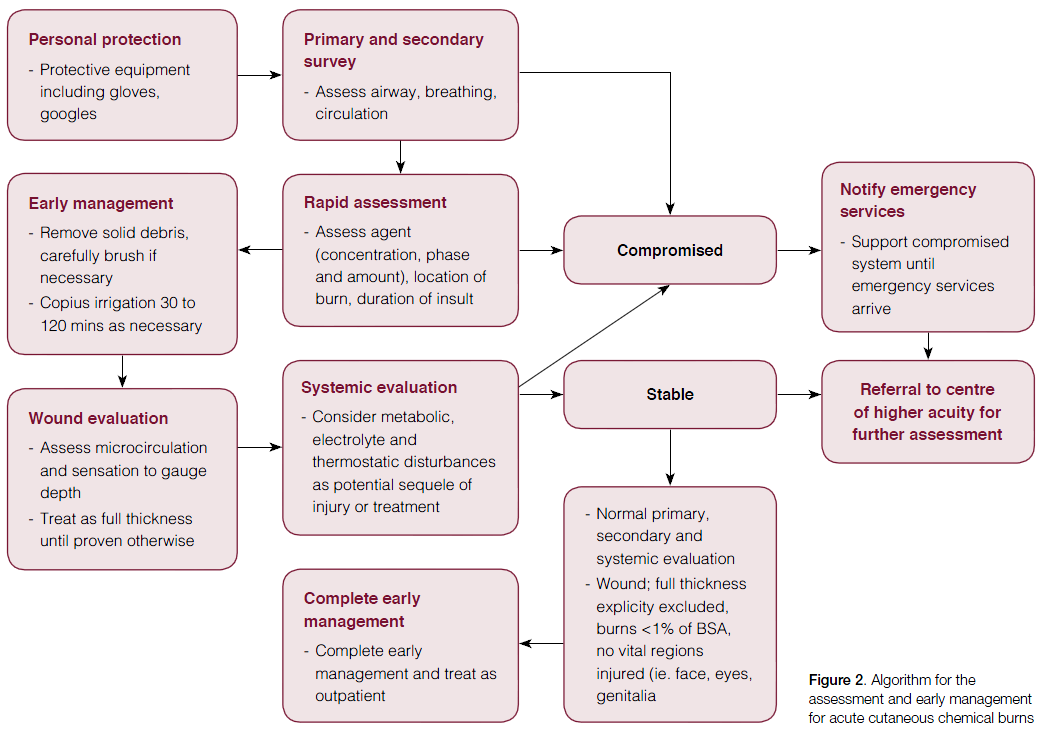
General prinicples of management
After primary survey and initial rapid assessment, the following care outlines the general principles for managing acute chemical burns.11
- Removal of the chemical: the duration of skin contact is the key determinant of injury severity.12,13 Thus, prompt removal of chemical contact is mandatory.
- This should be performed rapidly and generally requires removal of contaminated clothing at the scene of injury.14 Initially, residues or dust should be brushed off the skin.
- Irrigation should then be performed with warm water under a tap with appropriate drainage to prevent further injury. Care should be taken to ensure the wash off does not occur across unaffected skin. Early irrigation dilutes the chemical concentration and has been shown to reduce the severity of the burn and hospital stay.8 No objective measure for appropriate irrigation has been defined in the literature but it is widely accepted that 0.5–2 hours may be required to maintain a cutaneous pH of 5–11.10
- Neutralisation of chemicals is contentious but is generally not indicated because of the risk of further heat production and thus continuing injury. Several neutralising agents have shown some benefit,9 but irrigation with plain water remains the most efficacious, accessible and cost-effective treatment.12,15–17
- Complete wound evaluation: the microcirculation of the wound is evaluated by pinprick test for pain and capillary return time.18,19 Assessment regarding the depth of the chemical burn is notoriously difficult, as burns may be deceptively superficial.11,20 The difference in surface temperature between the affected and unaffected skin may assist in depth assessment.18,19 Re-assessment should be done at regular intervals as this may provide information about injury progression. As a general rule, unless the observer can be absolutely sure, chemical burns should be considered deep dermal of full-thickness until proven otherwise.
- Chemicals causing liquefactive necrosis, typically basic solutions, may cause continuing necrosis dispute removal of agent. Caution should be practised in such situations and expert opinion may be required.
- Debridement of blisters and non-viable tissue is advocated as early as possible via surgical or non-surgical approaches.7,21
- Systemic toxicity: the insulting chemical injury or subsequent treatment may produce systemic changes that require assessment and intervention.
- Metabolic disturbances: the most common disturbance is acid-base imbalance. Monitoring blood gases through venous sampling may be necessary to ensure metabolic stability.10,22
- Electrolyte disturbances and associated sequel: various chemicals may cause biochemical disturbances. As such, patients may require biochemical analysis on admission to higher acuity centres. For example, hydrofluoric acid (HFA) may cause hypocalcaemia and resulting cardiac arrhythmia.
- Hypothermia: may occur from the prolonged duration of wound lavage. Water temperature should be maintained as close to body temperature as possible.10
- Referral: Given the difficulty in assessing injury extent and depth, caution is generally advised. Chemical burns should be treated as full-thickness burns until proven otherwise.
- Unless full thickness burns can be explicitly excluded by the treating physician, referral to secondary or tertiary centres is required for formal assessment by specialist services. Full-thickness chemical burns may require admission for surgical debridement and grafting of non-viable tissue.
- Ocular chemical injury is beyond the scope of this review, but generally requires urgent ophthalmic review.
Specific agents
Table 1. Common domestic agents and mechanism of injury
|
Domestic item
| Chemical agent |
Pathological process
|
|---|
|
In the garage
|
|
Batteries (car)
|
|
Potent acid causing coagulative necrosis
|
|
In the laundry
|
|
Cleaners
|
- Ammonia
- Sodium hypochlorite
|
Potent alkali causing oxidization and liquefactive necrosis
|
|
Bleach
|
|
As previous
|
|
Pool cleaner
|
|
As previous
|
|
In the kitchen
|
|
Oven cleaners
|
- Sodium (or potassium) hydroxide
|
Potent alkali causing oxidation and production of heat (exothermic)
|
|
In the bathroom
|
|
Toilet cleaner
|
- Precursors of sulohuric acid
- Hypochlorite
- Hydrochloric acid
|
Potent acids and alkalis as previous
|
|
Drain cleaner
|
- Sulphuric acid
- Sodium hydroxide
|
Potent acids and alkalis as previous
|
Management of specific chemical agents is complex and is generally advised in the emergency department following early management. Current Australian guidelines have been previously published.23 Table 1 outlines common domestic products and the harmful agents they contain, with more comprehensive lists easily accessible online.
- Cement: is a common cause of chemical burns. The main injury-causing agent in wet cement is calcium oxide and resulting hydroxyl ion.15 Cement has multiple mechanisms of action, but predominantly can be classed as an alkali. Injury is insidious, usually presenting several hours after injury.24 Copious irrigation and periodic wound assessment should be performed to exclude the requirement of surgical debridement.25
- Tar: in liquid form, tar is superheated and classically causes deep thermal and chemical burns. If not removed promptly, tar cools and causes liquefactive necrosis and adheres to skin. Adherent tar should not be removed in the pre-hospital setting and urgent referral is required as surgical debridement may be necessary. Various household items such as baby oil, mineral oils and butter may aid in tar removal.7,26
- Hydrocholoric and sulphuric acids: burns caused by these agents are among the most commonly treated chemical burns. Common household goods contain moderate concentrations of such agents or their immediate precursors. On contact, these agents donate protons and cause coagulative necrosis of the affected tissue.27,28 Immediate irrigation is recommended. Excision of non-viable tissue should be considered early in the course of injury.6,10
- HFA: a large proportion of the population is at risk from HFA given its widespread use in the household setting. HFA has the potential to cause significant local and systemic effects despite a small contact wound.10 The onset of local effects are dependent on the concentration of the HFA.29 The injury caused by HFA causes liquefactive necrosis and causes interruption in the surrounding cellular physiology. The injury results in hypocalcaemia and hypophosphataemia30–32 and, potentially, cardiac arrhythmia. The fluoride component is a metabolic toxin affecting nerve transmission.33 Haemodialysis and cation exchange resins have been reported for removal of absorbed fluoride.33,34 Copious irrigation and early referral are essential. Inactivation of the fluoride ion is necessary by topical preparations (eg quaternary ammonium products or calcium gels) or infiltrative preparations (eg calcium gluconate).
- Phosphoric acids: such chemicals are found in fertilisers and explosives, thus generally causing injury in the industrial setting. White phosphorus ignites in the presence of oxygen and thus immediate removal is necessary. Particles may be identified with the aid of ultraviolet light or 0.5% copper sulphate solution and should be removed from the wound. Copious irrigation should be performed and the patient should be transported with a wet towel covering the injury. Systemic consequences including hypocalcaemia, hyperphosphataemia and cardiac arrhythmia have been previously reported.35
- Alkali: act as proton acceptors and classically cause progressing injury despite the removal of the harmful agent. As discussed above, alkalis cause liquefactive necrosis, allowing progression to deeper tissues. Initially alkali burns seem superficial, but may progress to full thickness within 48–72 hours.36 Brushing of residues and irrigation provides early control.10 Referral to a centre of higher acuity and consideration for wound debridement should be done given the nature of continuing injury.10 Common harmful alkali agents include sodium, ammonium, calcium and potassium salts.
Conclusion
Chemical burns are common and may cause significant physical, psychological and economic burdens on patients. Complete rapid assessment of the cutaneous injury and clinical status of the patient is essential in establishing the need for prompt referral to centres of higher acuity. Appropriate early management is crucial in reducing the period of patient morbidity. Current guidelines suggest water irrigation is the safest, most efficacious and readily available treatment option in the early stages of care of chemical burns.
Competing interests: None.
Provenance and peer review: Not commissioned, externally peer reviewed.