Case
Joe, 66 years of age, was referred with a 2-week history of a right upper eyelid abnormality. He complained of associated diplopia initially, but this subjectively improved after a few days as the eye became more difficult to open. He had a mild, intermittent headache for 4 weeks, relieved with oral paracetamol. There were no other neurological symptoms. He had no other symptoms of giant cell arteritis. His past medical history included hypercholesterolaemia for which he was taking regular statin therapy. He was an ex-smoker with a 40 pack-year history.
Initially, Joe’s general practitioner (GP) diagnosed conjunctivitis and prescribed chloramphenicol drops four times daily. One week later the symptoms had not improved and Joe was referred to the eye clinic complaining of increasing right periocular pain. On examination, his visual acuity was 6/5 in each eye. There was near complete ptosis of the right eye. The right pupil was fixed and dilated, and there was no direct or consensual light reflex present. Cover testing revealed right exotropia and hypotropia, while extraocular movements showed limited right adduction and elevation. These findings were consistent with a right, partial, pupil-involving oculomotor nerve palsy.
Question 1
What is the course of the oculomotor nerve?
Question 2
What are the causes of an isolated oculomotor nerve palsy?
Question 3
What clinical features raise the index of suspicion of a compressive lesion?
Question 4
What investigation does this patient urgently require?
Case continued
Joe was referred for an urgent CT angiogram. This showed a large unruptured posterior communicating artery aneurysm (Figure 1). He was admitted under the neurosurgical team.
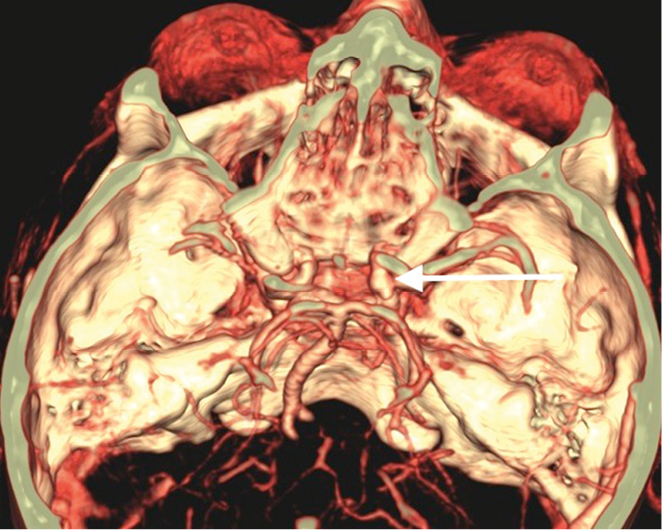 |
| Figure 1. 3-dimensional reconstruction image of the CT-angiogram showing an unruptured aneurysm of the posterior communicating artery (white arrow) |
Question 5
What are the surgical options for dealing with an unruptured intracranial aneurysm?
Question 6
What are the important learning points from this case?
Answer 1
The oculomotor nerve begins as several nuclei in the dorsal midbrain at the level of the superior colliculus. The fascicle emerges from the midbrain and passes into the interpeduncular space. The basilar portion runs parallel to the posterior communicating artery in the subarachnoid space. The nerve then enters the cavernous sinus by piercing the dura, and divides into superior and inferior branches, which enter the orbit through the superior orbital fissure.
The superior division innervates the levator palpebrae superioris and superior rectus, while the inferior division innervates the medial rectus, inferior rectus and inferior oblique muscles. The branch to inferior oblique also contains preganglionic parasympathetic fibres that innervate the ciliary muscle and spincter pupillae.
Answer 2
There are numerous causes of isolated oculomotor nerve palsy. Some of the more common causes are listed in Table 1.1 Microvascular ischaemic disease due to cardiovascular risk factors is one of the most common causes. However, it is important to consider and exclude potentially life-threatening causes such as giant cell arteritis and intracerebral artery aneurysm.
Up to 30% of cases of oculomotor nerve palsy are due to intracerebral aneurysm.2 The most common location is at the junction of the posterior communicating artery and the internal carotid artery. It is imperative to promptly diagnose these patients, as missing an aneurysm that subsequently ruptures carries a significant risk for morbidity and mortality. The average time from the onset of a third nerve palsy due to an unruptured posterior communicating artery aneurysm to major subarachnoid haemorrhage has been reported as 29 days.3
Table 1. Common causes of oculomoter nerve palsy
|
|
Intracerebral aneurysm
|
|
Infarction
|
|
Diabetes/microvascular damage
|
|
Cavernous sinus lesion
|
|
Trauma
|
|
Meningitis
|
|
Giant cell arteritis
|
Answer 3
The presence of pupillary involvement raises the index of suspicion for a compressive aneurysmal lesion, due to the characteristic superficial location of the pupillomotor fibres supplied by pial blood vessels. By contrast, the microangiopathy associated with microvascular disease affects the vasa nervorum blood supply, causing ischaemia of the main trunk of the nerve, while generally sparing the superficial pupillomotor fibres.
Pain is another important feature of many third nerve palsies due to posterior communicating artery aneurysm. Presentation is typically with an ipsilateral frontal headache.
It is important to remember that these features are not definitive. There have been several case reports of patients with a third nerve palsy due to aneurysm that presented with a normally reactive pupil.4 If a pupil-sparing third nerve palsy is not imaged initially, it is necessary to monitor closely to ensure the pupil does not become involved at a later stage. Similarly, pain is not universal in aneurysmal compression.
Answer 4
Formal catheter angiography has been considered the gold standard for the diagnosis of cerebral aneurysms. However, it may be associated with a 1–2% risk of systemic or neurological complications.5 With an experienced neuro-radiologist interpreting the images, modern computed tomography angiography (CTA) and magnetic resonance angiography scans should detect nearly all aneurysms responsible for an isolated oculomotor nerve palsy.6 Many clinicians prefer CT/CTA for the initial study because of its relatively easier accessibility and rapid acquisition time, although this varies depending on the institution.7
Answer 5
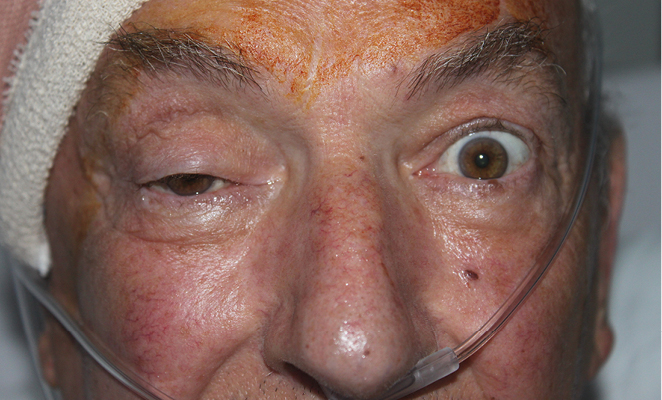 |
| Figure 2. Post-operative picture following microvascular clipping of the intracranial aneurysm Note the near-complete right ptosis |
The two main neurosurgical options are an endovascular coiling procedure or microsurgical clipping of the aneurysm. A study retrospectively analysed outcomes for oculomotor palsy recovery from seven patients who underwent endovascular coiling, compared with 10 patients who underwent clipping.8 There was no reported difference between the two groups.
Often the decision is based on a variety of surgical factors. In this case craniotomy with clipping was deemed the preferred option. Joe underwent this operation the following morning. The same afternoon he was recovering well (Figure 2). Note the near complete right ptosis persists.
Answer 6
Table 2. Important learning points from this case
|
|
Neurogenic ptosis should not be confused with eyelid swelling from conjunctivitis or periorbital infection
|
|
A third nerve palsy will often present with ptosis, and the globe may be in an abducted and depressed position: ‘down and out’
|
|
When assessing a patient with either ptosis or diplopia, it is imperative to check that the pupils are equal in size and react equally to light
|
|
Urgent referral is crucial if there are signs of a third nerve palsy, and neuro-imaging may be required to investigate for an intracranial aneurysm
|
|
It is important to ask about symptoms of giant cell arteritis if the patient is 50 years or older
|
There are several important lessons to be learned from this case. These are summarised in Table 2. It is important to be able to differentiate eyelid ptosis from eyelid swelling, which may be the result of an infective process such as conjunctivitis. Viral conjunctivitis is typically associated with conjunctival hyperaemia, watery discharge, conjunctival follicles and pre-auricular lymphadenopathy, and is often bilateral. Eyelid ptosis is defined as less than 2 mm between the upper eyelid margin and pupil light reflex, or an asymmetry of more than 2 mm between the eyes.9 Important causes include congenital ptosis, levator aponeurosis dehiscence, Horner’s syndrome, myasthenia gravis and oculomotor nerve palsy. In any patient with possible eyelid ptosis, it is mandatory to lift the ptotic lid and assess pupillary light reflexes and extraocular movements. Any patient with anisocoria or restricted eye movements warrants urgent referral.
Competing interests: None.
Provenance and peer review: Not commissioned, externally peer reviewed.