Good control of blood glucose levels is known to be associated with reduced long-term complications; however, this control is difficult to achieve in many patients, particularly those with type 1 diabetes. From the landmark Diabetes Control and Complications Trial (DCCT), multiple daily insulin injections (MDI) has become the treatment of choice for type 1 diabetes. Together with the use of insulin analogues, this is recognised as state-of-the-art treatment; however, most patients do not achieve the target glycosylated haemoglobin (HbA1c) level of <7%, and about 20% of patients with type 1 diabetes experience episodes of severe hypoglycaemia at a frequency of about 1 per patient per year.
Insulin pump therapy or continuous subcutaneous insulin infusion (CSII) was introduced in the 1980s in an attempt to improve outcomes in patients with type 1 diabetes. As the name suggests, these pumps have been designed to infuse insulin subcutaneously and are able to provide a background or basal insulin infusion in association with bolus doses that can be administered with food or to correct high blood glucose levels (Figure 1). Bolus doses may also be a fraction of a unit allowing for finer dose adjustments. In theory, infusion of rapid-acting insulin into one site should have reduced variability, compared with multiple injections into different sites. Furthermore, CSII introduces the possibility of varying the background or basal infusion rates according to patient needs, and having as many bolus doses of rapid insulin that may be needed to correct for high readings or for added unscheduled snacks.
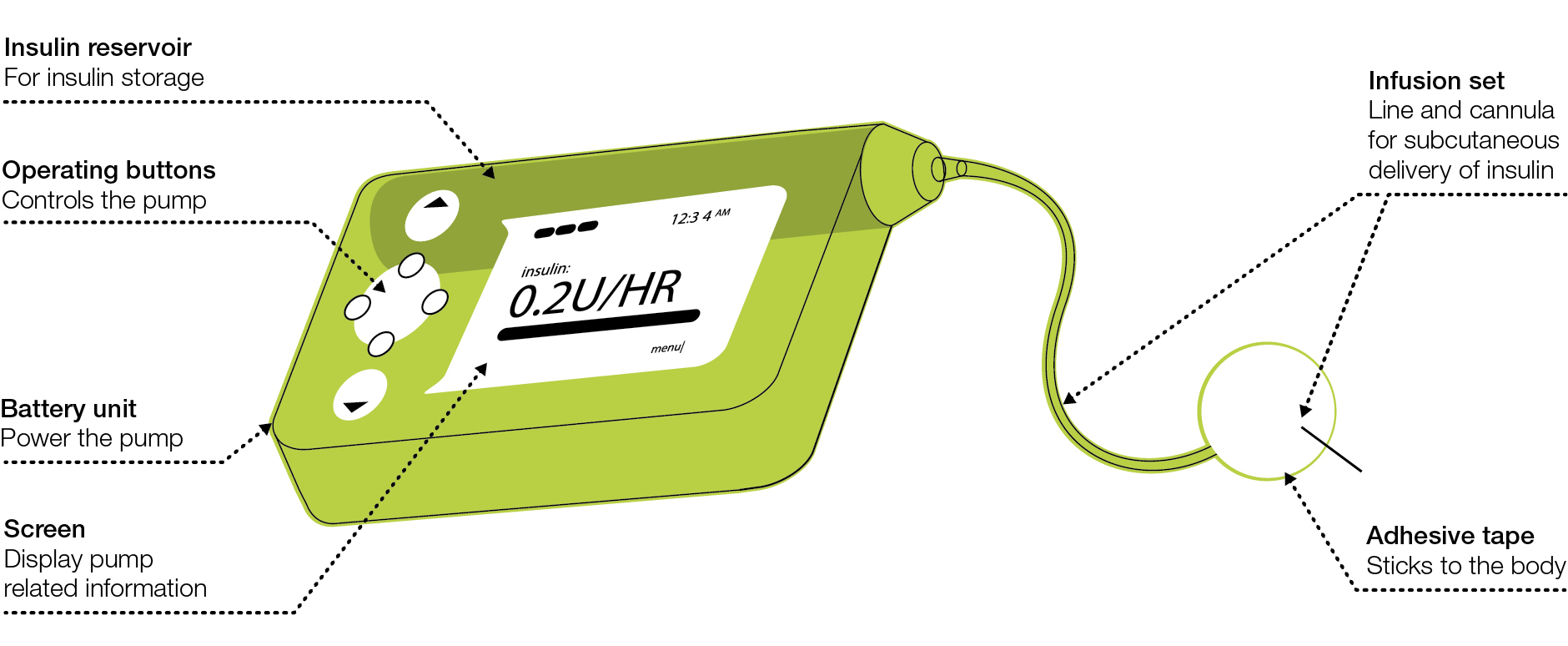 |
Figure 1. Continuous subcutaneous insulin infusion pump
Reproduced with permission from Diabetes Australia – Victoria, from Understanding insulin pumps: information for people with type 1 diabetes.
Melbourne: Diabetes Australia Vic, 2013. |
CSII has developed over the years so that there are a number of different models available with functions such as extended boluses, temporary basal rates, and more compact ‘patch’ pumps that do not require tubing. These pumps need to be worn 24 hours a day and the cannula site changed every 3 days. Although pumps are worn continuously, there are now accessories that allow for more discreet wearing of pumps under clothing, and advances that allow for less handling of the pump and remote activation.
CSII has improved outcomes for patients in terms of hypoglycaemia reduction, HbA1c improvement and quality of life, but there is a limit to the benefits, and some patients continue to struggle to achieve optimal control.1 One of the limitations of CSII is that subcutaneous insulin administration is peripheral, whereas pancreatic insulin, which involves the portal system, has important effects on hepatic glucose metabolism. Furthermore, subcutaneous insulin absorption is slow, compared with the fast onset and offset of normal beta cell function. Furthermore, the requirement for regular blood glucose testing is no different with CSII, compared with standard MDI.
Subcutaneous CGM has been developed and now has proven benefit in type 1 diabetes.2 It involves the subcutaneous insertion of a glucose sensor attached to a transmitter that sends signals to either an insulin pump or a hand-held meter. These are worn for 7 days with the sensor inserted into subcutaneous abdominal fat. Most of these devices need regular calibration and blood glucose testing about twice a day. The accuracy of these devices has been an issue, but they have been improved in recent years. Subcutaneous glucose levels change more slowly than plasma glucose, and this may be an important limitation, particularly if glucose levels are changing rapidly. Subcutaneous glucose levels, therefore, have a short time lag, compared with blood glucose measurements, and measurements may not always match blood glucose. Nevertheless, when worn regularly (changed every 7 days), they improve outcomes in terms of hypoglycaemia and hyperglycaemia. As the devices and associated pumps have advanced, patients can now be alerted to hypo – and hyperglycaemia, and take early action to correct blood glucose levels. If worn in association with one of the new Medtronic Veo pumps, a low glucose level identified on CGM will not only alert the patient through an alarm system, but shut the pump off until glucose levels recover. This is known as the ‘low glucose suspend’ feature.
CGM is also available as a 1-week diagnostic test in which the patient is blinded to glucose levels at the time and the sensor is downloaded at the end of the week. The literature behind this type of retrospective or blinded CGM is not as strong as with the real-time CGM, but it can be a valuable tool in both type 1 and type 2 diabetes, and can be used in primary care.
Identifying the patient who might benefit from CSII
Most patients with type 1 diabetes are adequately maintained on MDI together with insulin adjustment for carbohydrate content, exercise and acute illness. In Australia, 10% of patients with type 1 diabetes are treated with CSII; internationally, figures vary from 25% to less than 5%. In the majority of cases, pump use commences in childhood, although there are growing numbers of users commencing as adults.
Many paediatricians and parents prefer children and adolescents to use pumps because of the flexibility and improved control associated with CSII, particularly in view of the erratic lifestyle and growth issues in these age groups. In adults with established type 1 diabetes, indications may vary and include lifestyle and quality-of-life factors, and regular hypoglycaemia on MDI therapy. Many people with type 1 diabetes have impaired quality of life associated with frequent hypoglycaemia, erratic blood glucose levels and fear of hypoglycaemia. A smaller percentage of patients experience frequent episodes of severe hypoglycaemia that require assistance or hospitalisation. These are examples of circumstances in which CSII should be considered and discussed. Some degree of technological capability is required to run a pump, so elderly patients with type 1 are less likely to start using a pump. Patients with type 1 diabetes preparing for pregnancy may decide to trial CSII before pregnancy in order to achieve and maintain better control during pregnancy. In all cases, patients need to have a high level of compliance with monitoring glucose frequently, as well as having an adequate grasp of carbohydrate counting.
What is required for a pump start
Pump starts require a specialist multidisciplinary team including an endocrinologist/paediatrician, credentialled diabetes nurse educator and a dietician. Pre-pump education includes:
- assessment of the indication and patient expectations
- dietician review and initiation or reinforcement of carbohydrate counting
- discussions regarding pump types and general workings of pumps re-siting, adjusting rates and problem solving.3
In the event of rapidly rising blood glucose levels, patients need to have an action plan including the ability to re-site cannulae, as one of the common problems encountered is kinking or blocking. Patients need to be aware of the costs involved. Pumps cost more than $8000. This cost is covered by most private health insurance policies with hospital cover. The accessories – tubing, cannulae – are subsidised by the National Diabetes Services Scheme (NDSS) and therefore affordable.
Pump starts are usually, but not always, performed in a hospital setting. Day admission may be required and the patient can expect to stay for at least 4–6 hours on the first day. The next few weeks require regular follow-up and adjustments with the team, and after-hours numbers must be available for problem solving in the event of unstable blood glucose levels. Many of the modern pumps have software that patients can use to download their data and send it to the team for assessment and adjustments (Figure 2). Patients need to be aware that pumps may fail at a rate of about 1 in 5 over 4 years, so clinicians must inform patients that they might need to restart MDI in case of an emergency.
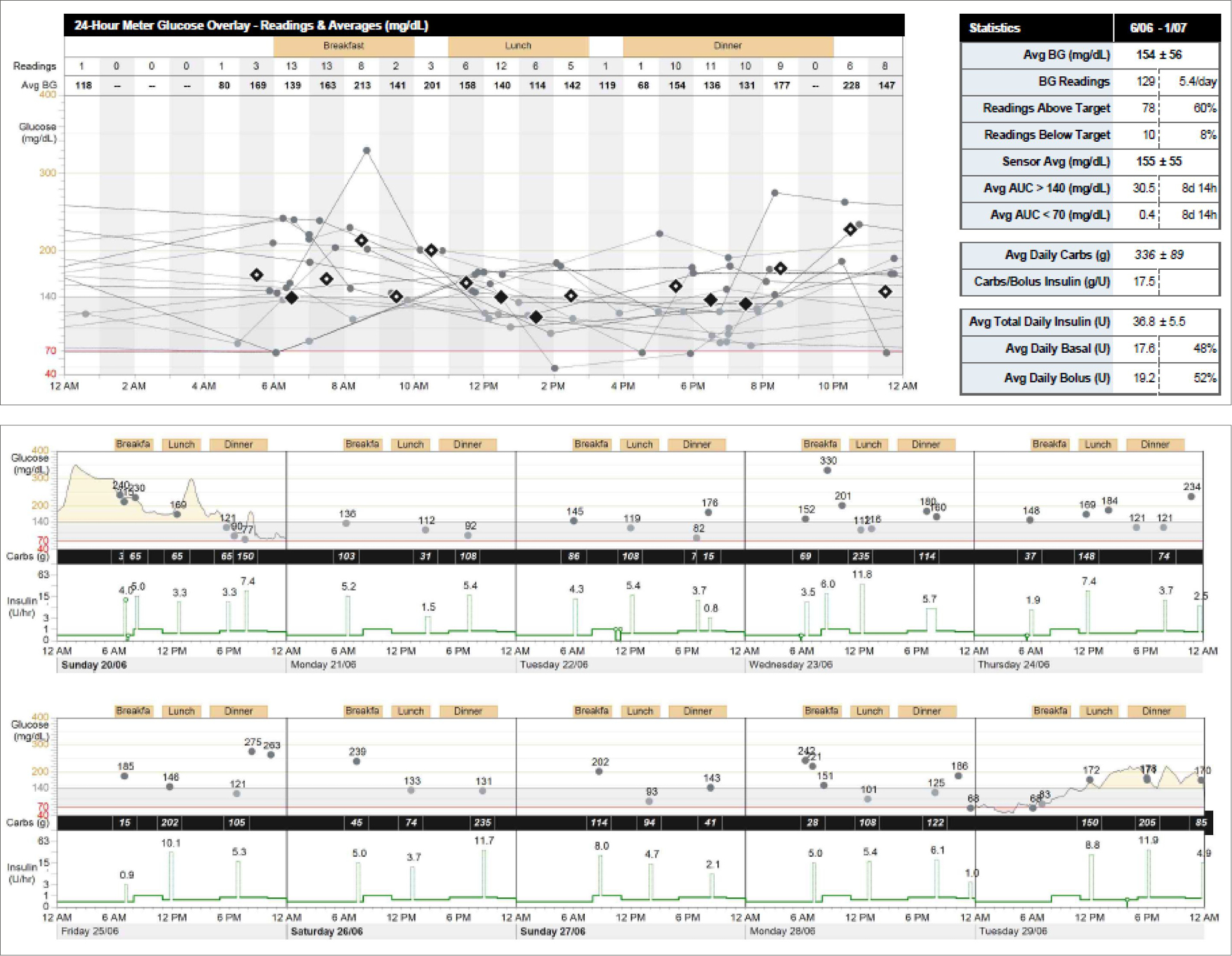 |
| Figure 2. Insulin pump download featuring blood glucose readings, carbohydrate count and insulin doses |
When is CGM indicated?
CGM is a useful tool in pump users, non-pump users with
type 1 diabetes, and those with complex type 2 diabetes. The most commonly used CGM is in combination with CSII, and most modern pumps allow for CGM to be read in real time on the pump. CGM can also be used in non-pump users who purchase the hand-held meter. CGM removes the need for frequent fingerpricking; however, current CGM requires calibration with a minimum of two fingerprick measurements per day. Many patients are concerned about severe, nocturnal hypoglycaemia, and CGM should be considered for high-risk patients, given the morbidity and possible mortality associated with severe nocturnal hypos. High-risk groups include patients with hypoglycaemia unawareness and particularly ‘frequent flyers’ with regular severe hypos. Another important group is the patient with type 1 diabetes who is pregnant and aims for meticulous glucose control. CGM should be considered in patients who have occupations that require warning of hypoglycaemia such as professional drivers, or those working in remote or offshore environments.
Unfortunately, CGM is expensive and has no government support in Australia at this time. The transmitter costs at least several hundred dollars, and each sensor is around $70 for 1 week. Worn full-time, CGM would cost over $3000 per year. This has limited its use in Australia and many countries around the world. CGM can be worn part-time, for example, 1 in 4 weeks, which limits the cost. The evidence, however, is that the more frequently it is worn, the more benefit is derived from wearing it.
Retrospective CGM is an important tool that is used regularly by clinicians to identify glucose patterns in patients with more complex type 2 and type 1 diabetes. Many specialist diabetes clinics have purchased transmitters and are able to provide this service to identify highs and lows that occur regularly. CGM is inserted at the clinic, removed at the end of the week and downloaded for the patient and clinician to view. Although largely confined to specialist clinics, a recent Australian study has shown the benefits of using this technology in primary care.4 This should be considered in patients on complex therapies not achieving adequate control, particularly in those not testing glucose very frequently. Again, if there is a concern about nocturnal hypoglycaemia, CGM is an important tool for reassuring patients or informing them that they are at risk.
The future of pumps and CGM
Pump therapy and CGM has now advanced to the point where the next step is closing the loop. The key limitations are the accuracy of CGM, the slowness of subcutaneous rapid-acting insulin, compared with normal beta cell response, and the usability of complex computer algorithms required to safely adjust insulin infusion rates. There are trials currently underway in Australia and around the world looking at the safety and efficacy of closed-loop systems.5 No doubt we will see the artificial pancreas become a reality in the next few years, and this is likely to dramatically change the lives of our patients with type 1 diabetes in terms of complication rates and quality of life.
Key points
- Insulin pump therapy reduces hypoglycaemia and improves HbA1c, compared with MDI.
- Insulin pump therapy may be indicated in patients with
type 1 diabetes and inadequate glycaemic control or at high risk of severe hypoglycaemia.
- Insulin pump therapy requires a high level of motivation, compliance and some technical capability.
- CGM may benefit patients at high risk of severe hypoglycaemia, or in patients requiring meticulous glycaemic control.
Competing interests: Neale Cohen’s institution has received grants from Sanofi and Novartis. He has also received consulting fees from Abbott, Lilly, Boerhinger-Ingelhein, Astra Zeneca, Sanofi, Medtronic and Merck. Lilly, Novartis and Novo Nordisk have also provided support for travel to meetings or other purposes.
Provenance and peer review: Commissioned, externally peer reviewed.