Extensive literature spanning several decades exists on the concept of ‘diagnostic delay’ in cancer.4,5 This recognises that patient pathways to presentation to healthcare and initial management in primary care are key determinants of cancer patient outcomes.6–8 At a community level, symptom awareness campaigns can promote earlier presentation to primary care9 and GPs can further reinforce these messages, especially for those who may be at higher risk of developing cancer.10 Reducing diagnostic delays, such as the time between the first symptomatic presentation by a patient to their GP and their first specialist referral for further investigation,11 could lead to earlier stage diagnosis and improved outcomes.12 Perceptions of delay in cancer diagnosis are a leading cause of medico-legal claims in general practice,13 highlighting how important early recognition of cancer is from a patient perspective even where there may be doubt about how much an earlier diagnosis would have affected prognosis.
Symptoms of cancer in general practice
Diagnosing cancer in general practice is not easy and some cancers are more difficult than others to diagnose when they present first in primary care. A large UK study showed that patients with lung, pancreatic and stomach cancer, and myeloma were significantly more likely to have had multiple visits to their GP before referral, compared with patients with breast or endometrial cancer.14 This suggests that GPs took longer to recognise the significance of their patients’ symptoms for certain types of cancer. It also highlights the importance of repeat visits with the same symptoms as a potential red flag in itself and the value of diagnostic safety-netting to ensure that patients know they need to return if their symptoms persist.15
A major challenge for GPs is that the symptoms of many cancers are common in the community and overlap with more prevalent benign conditions. In a Danish study, only about half of all cancer patients presented with classical alarm symptoms in general practice.16 The gatekeeper role of general practice in Australia means that GPs need to assess a patient’s current symptoms as well as their underlying cancer risk factors to determine whether further investigation or referral for suspected cancer is required.17
In recognition of the challenges in diagnosing cancer in general practice, many national and international guidelines have been published, which summarise the symptoms of cancer that are of greatest significance and require urgent investigation.18,19 For example, Cancer Australia has published guidelines for the investigation of suspected breast, ovarian and lung cancer;20–22 (Table 1) some state health departments in Australia have also developed cancer referral guidelines.23
There is growing interest nationally in fast-track referral routes for suspected cancer, particularly within public health systems where there may be long waiting times for certain diagnostic tests such as colonoscopy or upper gastrointestinal endoscopy. In Australia open-access gastrointestinal endoscopy has been introduced in several states to reduce potential diagnostic delay, some of which explicitly apply specific fast-track referral criteria. International evidence suggests that fast-track referral routes may have some value in improving early diagnosis of cancer but there is significant variation in how individual GPs use these rapid access pathways.24 Implementing fast-track diagnostic routes to improve cancer diagnosis requires a significant investment in implementing the underlying diagnostic guidelines. This raises the question of how strong the evidence is on which symptoms best predict cancer, as it presents in primary care, to inform case selection for urgent investigation.
Predicting risk of undiagnosed cancer in general practice
Currently many cancer diagnostic referral guidelines for general practice depend mostly on the presence of single symptoms as opposed to clusters of symptoms. Furthermore, many of the existing guidelines are based on studies of referred populations, which tend to overestimate the predictive value of single symptoms. Single symptoms are relatively poor predictors of cancer in primary care and some patients will not present with symptoms that actually warrant an urgent referral.3 Critically, even so-called red-flag symptoms such as rectal bleeding and weight loss have positive predictive values (PPV) of less than 5% in unselected primary care populations.25
In the last few years a growing body of evidence has developed on how well symptoms predict cancer in primary care. Importantly, this evidence has been derived from very large, validated general practice databases enabling predictive models to be developed. Although all this research has been conducted in the UK, it is highly unlikely that common cancers would present differently in Australian general practice, in terms of their symptom profile. Moreover, in Australia we simply do not have comparable large general practice datasets to develop our own cancer diagnostic models. These cancer diagnostic models, therefore, represent the best evidence available about how symptoms in general practice predict an undiagnosed cancer.
Hamilton’s CAPER studies have developed a series of risk assessment tools for specific cancers, which provide the PPVs for single and pairs of symptoms, signs or common investigations.26–32 These were originally summarised in the form of risk charts (Figure 1a, b). The colour-coding is used to classify patients at different cut-offs of risk of an undiagnosed cancer, with a PPV ≥5% suggested as requiring urgent investigation.
Table 1. Examples of symptoms and signs associated with cancers from Australian guidelines
| Lung cancer22 |
|---|
| • Unexplained haemoptysis |
| • Unexplained or persistent for >3 weeks: |
| –New or changed cough |
| –Chest and/or shoulder pain |
| –Shortness of breath |
| –Hoarseness |
| –Weight loss/loss of appetite |
| –Unresolved chest infection |
| • Abnormal chest signs |
| • Finger clubbing |
| • Cervical and/or supraclavicular lymphadenopathy |
| • Signs of pleural effusion |
| Ovarian cancer22 |
| • Abdominal bloating/feeling full* |
| • Appetite loss |
| • Unexplained weight loss |
| • Constipation |
| • Heartburn |
| • Back pain |
| • Frequent urination |
| • Abdominal/pelvic pain |
| • Fatigue |
| *Persistent abdominal bloating (ie distension) is much more strongly associated with ovarian cancer than intermittent bloating40 |
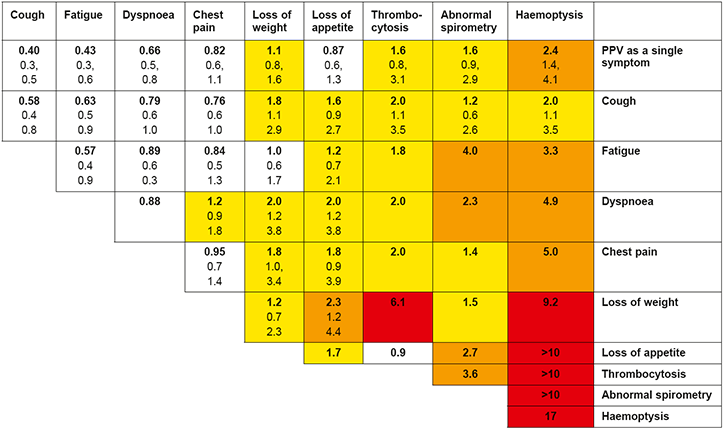
Figure 1a. CAPER risk chart for lung cancer Positive predictive values (%) for lung cancer for individual risk markers and pairs of risk markers in combination (against a background risk of 0.18%).
Notes: (1) The top row gives the positive predictive value (PPV) for an individual feature. The cells along the diagonal relate to the PPV when the same feature has been reported twice. Other cells show the PPV when a patient has two different features. (2) The top figure in each cell (in bold) is the PPV. The two other features are the 95% confidence intervals for the PPV. These have not been calculated when any cell in the 2 × 2 tables was below 10. (3) The yellow shading is when the PPV is above 1%. The amber shading is when the PPV is above 2%. The red shading is for PPVs above 5.0%.
Reproduced with permission from the BMJ Group from Hamilton W, Peters TJ, Round A, Sharp D. What are the clinical features of lung cancer before the diagnosis is made? A population based case-control study. Thorax 2005;60:1059–65.
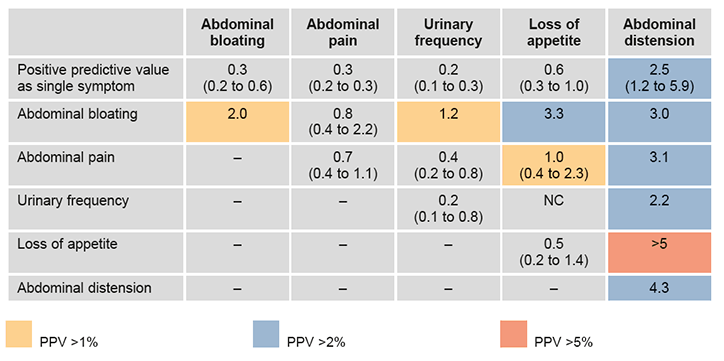
Figure 1b. CAPER risk chart for ovarian cancer. PPVs (95% confidence intervals) for ovarian cancer for individual risk markers and for pairs of risk markers in combination (against background risk of 0.04%). Reproduced with permission from the BMJ Group from Hamilton W, Peters TJ, Bankhead C, Sharp D. Risk of ovarian cancer in women with symptoms in primary care: population based case-control study. BMJ 2009;339:b2298.
One of the limitations of the CAPER charts is that they do not account for individual baseline risk factors, such as age, family history or smoking, although separate lung cancer charts exist for smokers and non-smokers.
Hippisley-Cox has developed slightly more sophisticated cancer diagnostic risk models, called QCancer. There are separate models for men and women, which predict risks of a range of different cancers according to baseline risk factors, current symptoms and specific clinical conditions such as anaemia and venous thromboembolism.33,34 Importantly, these models account for age, smoking, alcohol and, to a limited extent, family history of cancer. While these risk factors are often considered more in the context of cancer prevention and predicting future risk of cancer,35 they are also relevant in the assessment of risk of an undiagnosed cancer in the presence of symptoms. The QCancer models have potentially greater clinical relevance because, as one would expect, common symptoms are associated with more than one type of cancer. For instance, abdominal pain is associated with eight and nine different cancers in men and women respectively; a change in bowel habit is associated with four cancers in women. QCancer has been developed into an online risk calculator that is freely available at www.qcancer.org
Issues for the future
At present, diagnosing cancer in primary care will continue to depend on GPs’ clinical acumen and careful case selection of patients whose symptoms are more strongly suggestive of cancer. GPs traditionally apply heuristic approaches to diagnosing cancer, based on the pattern of symptoms, signs, other risk factors and knowledge of the individual patient and their previous consulting patterns. Heuristic approaches to diagnosis are well recognised as prone to a range of biases,36 although a recent large Danish study suggests that GP suspicion of a serious diagnosis, including cancer, has reasonable predictive value.37 The cost-effectiveness of the Australian healthcare system depends on GPs avoiding ‘over-investigation’ of patients at very low risk of an undiagnosed cancer and the consequent unnecessary burden on patients and potential impact on access to diagnostic tests.38 Despite that, GPs will tend to have a low threshold for investigation or referral because of the threat, both clinically and medico-legally, of a later diagnosis. While we await the development of better biomarkers to help diagnose cancer earlier, GPs will need to apply the existing evidence on symptoms and how they predict risk of undiagnosed cancer.
However, we need to understand more about how risk assessment tools, such as the Hamilton and QCancer models, can be integrated into routine practice. Cardiovascular risk calculators have been gradually integrated into routine use to support targeted prevention and prescribing but it is unclear how use of cardiovascular risk predictors maps onto use of cancer risk tools in general practice. In the UK there are ongoing pilot projects of electronic implementation of the Hamilton and QCancer risk tools within the GPs’ clinical software. But we do not know yet how best to use these in the consultation or, alternatively, as some form of audit tool to flag patients who may require investigation. Our own research is exploring some of these issues for Australian general practice, initially exploring how GPs might use them in consultations to assess symptoms and determine selection of diagnostic tests. Further consideration is also required about what level of risk warrants urgent investigation and, potentially, access to a fast-track referral pathway. Is a 5% cut-off too conservative given the potential implications for delayed diagnosis? GPs are not used to considering absolute risks of a diagnosis when making decisions about investigation and referral, at least not in such an explicit way but maybe this approach needs further consideration to optimise appropriate investigation and referral of patients who are more likely to have an undiagnosed cancer.
At an individual GP level, a cancer diagnosis is made approximately only 7–8 times each year,39 yet GPs see patients with symptoms that could be due to cancer on a daily basis. Herein lies the challenge for general practice in diagnosing cancer early.
Competing interests: None.
Provenance and peer review: Commissioned, externally peer reviewed.