Chronic heart failure is a clinical syndrome associated with unpleasant symptoms such as shortness of breath, ankle swelling and fatigue.1 In Australia, the National Health Survey reported an overall prevalence of 1.3% in the general population.2 It is uncommon before age 45 years, and then prevalence increases with each decade of life. In a large English population-based study that recruited 6162 participants, 2.3% of adults aged 45 years and over had heart failure.3
Hospitalisation for patients with chronic heart failure is common, costly and associated with poor survival, both as an inpatient and in the months following discharge.4 However, evidence-based treatments are available to prevent hospital admission and improve outlook.5 Randomised controlled trials found that angiotensin converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), beta-blockers and aldosterone antagonists improved quality of life, reduced hospitalisation and increased survival of patients with heart failure with reduced ejection fraction (HFREF).6–8 Community-based heart failure programs also offer an opportunity for active management of patients to optimise chronic heart failure treatment.9
There is a paucity of recent data on chronic heart failure management in Australian general practice. Epidemiological information about chronic heart failure is mainly available from national surveys reliant on accuracy of self reporting,2 or large-scale screening studies where patients have voluntarily taken part in research.3 Data collected directly from general practice provide objective evidence of the current burden of chronic heart failure and how it is being managed.
The aim of this study was to describe the prevalence of chronic heart failure and the management of patients attending general practice in Australia, including natriuretic peptide test use, medication rates, hospitalisation and discharge to community-based programs in patients with chronic heart failure.
Methods
The study was undertaken through three Supplementary Analysis of Nominated Data (SAND) sub-studies of the Bettering the Evaluation and Care of Health (BEACH) program, a continuous, national, cross-sectional survey of Australian general practice activity. BEACH methods are described in detail elsewhere.10 Briefly, a random sample of approximately 1000 currently active general practitioners (GPs) are recruited each year. Each GP participant records details for 100 encounters with consenting, unidentified patients, on structured paper forms. Information is collected about what is managed for each patient at each visit on the days the GP is participating. Throughout the program, a series of SAND sub-studies use the GP as an ‘expert interviewer’ to record, in discussion with the patient, aspects of patient health additional to the content of the encounter. The three SAND data periods exploring chronic heart failure management were: period 1: November 2010 – January 2011 inclusive; period 2: March – May 2014; period 3: March – May 2015. For these three periods GPs were asked to answer questions about chronic heart failure on 30 consecutive bespoke recording forms in their pack of 100.
The number of patients who attended general practice at least once in 2014–15, by patient age–sex, was provided by the Australian Government Department of Health.10 To provide an estimate of chronic heart failure prevalence in the attending population, the age–sex distribution of patients surveyed in these three data periods was adjusted (weighted) to reflect that of total patient attendees. We assumed that those people who did not attend general practice at least once in 2014–15 did not have diagnosed chronic heart failure, so accordingly calculated the population prevalence of chronic heart failure in Australia.
Chronic heart failure diagnosis and medications (including ACE inhibitors, ARBs, beta-blockers, calcium channel blockers, diuretics, antithrombotic, anti-angina and lipid modification drugs) were collected in all study periods. Stage of chronic heart failure, classified using the New York Heart Association (NYHA) criteria I–IV, was collected in periods 1 and 3. Natriuretic peptide testing was collected only in period 3. GPs recorded whether patients with diagnosed chronic heart failure had been tested at the time of diagnosis or since their diagnosis. Initiation of chronic heart failure medication (by the GP or specialist) was collected for periods 2 and 3. Whether the patient had ever been hospitalised for chronic heart failure was collected in period 2, while hospitalisation for chronic heart failure in the previous 12 months was collected in period 3. Discharge to a community-based heart failure management program was surveyed in periods 2 and 3.
Proportions and robust 95% confidence intervals (CIs) were calculated using survey procedures in SAS (version 9.3)11 that adjust for the study’s cluster design. Statistical significance of differences was judged by non-overlapping 95% CIs, the criterion of which is equivalent to P <0.006, more conservative than the usual P <0.05.12
The BEACH program and all sub-studies have ethics approval and oversight from the Human Research Ethics Committee of the University of Sydney (reference 2012/130).
Results
Completed chronic heart failure questions were received for 8989 patients from 308 GPs. Of these patients, 324 had chronic heart failure, an overall prevalence of 3.6% (95% CI: 3.1–4.2). Chronic heart failure was similarly prevalent among men (4.4%; 95% CI: 3.5–5.3) and women (3.1%; 95% CI: 2.5–3.6). Prevalence of chronic heart failure among the sampled patients increased with age to peak at 13.9% among those aged 75 years and over (Table 1).
Table 1. Age-specific rate of chronic heart failure among general practice patients in the study sample
|
Age group (years)
|
Patients with diagnosed chronic heart failure (n)
|
Total sampled patients (n)
|
Age-specific prevalence 95% CI)
|
|---|
|
0–44
|
2
|
3,742
|
0.1 (0.0–0.1)
|
|
45–64
|
52
|
2,470
|
2.1 (1.5–2.7)
|
|
65–74
|
62
|
1,235
|
5.0 (3.6–6.4)
|
|
75+
|
206
|
1,478
|
13.9 (12.0–15.9)
|
|
Total*
|
324
|
8,925
|
3.6 (3.1–4.2)
|
|
*Age was missing for two chronic heart failure patients, so sum of age groups is less than total patients.
CI, confidence interval
|
The prevalence of chronic heart failure among patients who attended general practice at least once in the year was estimated to be 2.0% (95% CI: 1.7–2.4). When extrapolated to the entire Australian population, the prevalence of diagnosed chronic heart failure was estimated to be 1.8% (95% CI: 1.5–2.1).
Stage of disease (NYHA class I–IV) was recorded (in periods 1 and 3) for 216 patients. Chronic heart failure was recorded as class I for 54 patients (25.0%; 95% CI: 18.2–31.8), class II for 94 (43.5%; 95% CI: 35.8–51.2), class III for 50 (23.1%; 95% CI: 16.9–29.4) and class IV for 15 (6.9%; 95% CI: 2.7–11.2) (Figure 1). There were three patients with chronic heart failure (1.4%) for whom stage/class was not known.
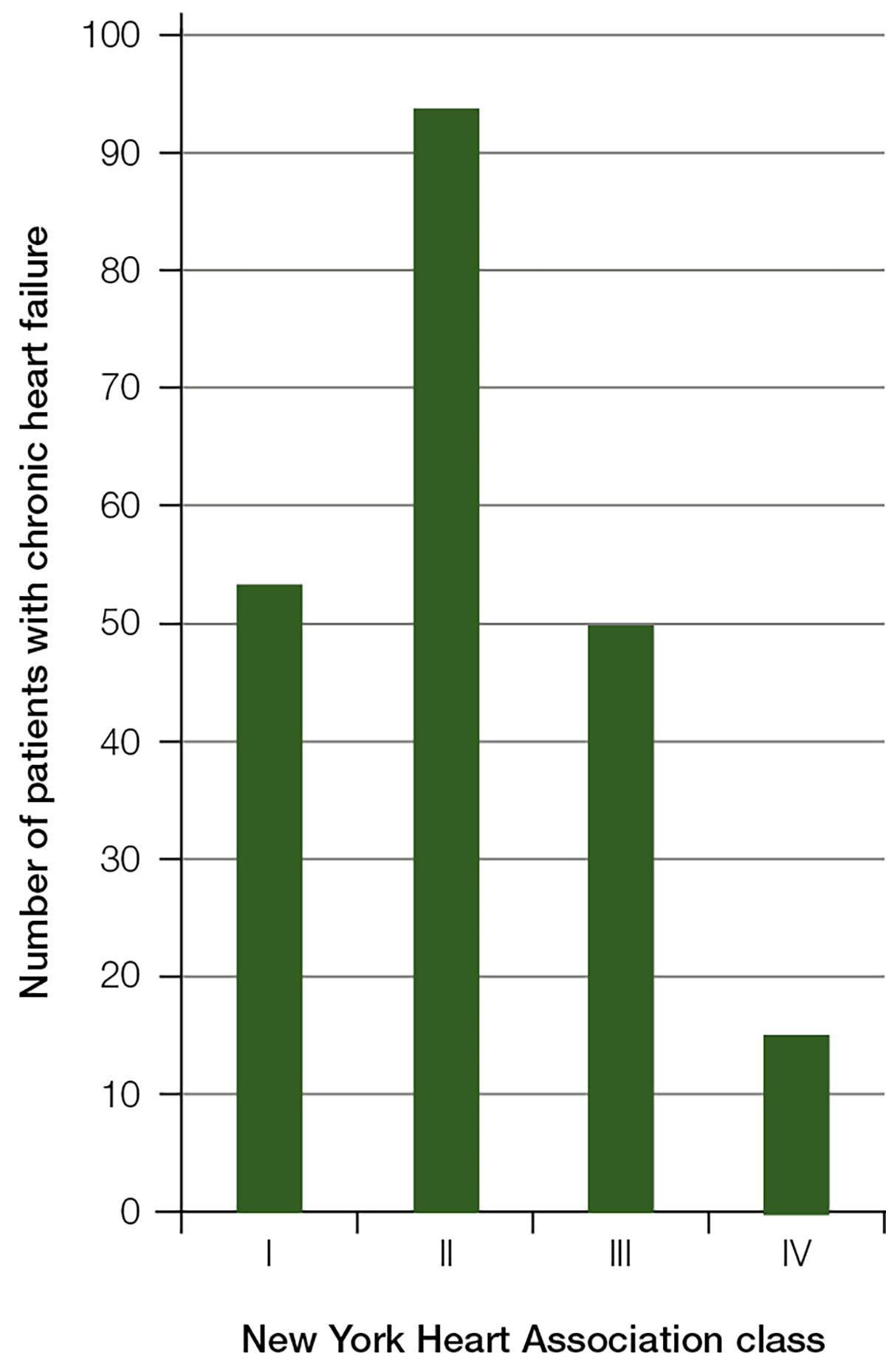
Figure 1. Number of patients with chronic heart failure by New York Heart Association class
Natriuretic peptides were explored in the period 3 cohort. Of the 103 patients with chronic heart failure, 96 responded to this question. Of these patients, five (5.2%; 95% CI: 1.0–9.4) had been tested at diagnosis, 11 (11.5%; 95% CI: 1.6–21.3) had been tested since diagnosis, 49 (51.0%; 95% CI: 38.3–63.8) had not been tested, and 31 (32.3%; 95% CI: 20.5–44.1) did not know if they had been tested. If only those patients who knew whether they had been tested were included in the analysis, 16 out of 65 chronic heart failure patients (24.6%; 95% CI: 7.4–41.8) had undergone natriuretic peptide testing.
Medication status was recorded for 313 of the 324 chronic heart failure patients. The vast majority (97.1%) were taking at least one medication agent for their heart failure (Table 2). The mean number of heart failure medication agents was 2.26 (95% CI: 2.13–2.39) per patient. In periods 2 and 3, the proportion of heart failure medication agents initiated by the GP, rather than a specialist, was 31.9% (95% CI: 24.8–39.1).
Table 2: Number of heart failure medication agents prescribed to patients with chronic heart failure
|
Number of heart failure medication agents
|
Number of patients
|
Percentage of total patients % (95% CI)
|
|---|
|
0
|
9
|
2.9 (1.1–4.7)
|
|
1
|
63
|
20.1 (14.8–25.5)
|
|
2
|
117
|
37.4 (32.2–42.6)
|
|
3
|
88
|
28.1 (22.7–33.6)
|
|
4
|
34
|
10.9 (7.1–14.6)
|
|
5
|
2
|
0.6 (0.0–1.5)
|
|
CI, confidence interval
|
|
|
Whether the patient was ever hospitalised for chronic heart failure was examined in period 2. Thirty-seven of 84 (44.0%; 95% CI: 34.5–53.6) patients with chronic heart failure had been hospitalised with an acute episode of heart failure. In 15 of 36 cases (41.7%; 95% CI: 22.1–61.3), the admission occurred via GP referral. The mean length of stay for the most recent admission was 6.5 days (95% CI: 4.4–8.7).
In period 3, hospitalisation in the previous 12 months was explored. Of the 103 patients with chronic heart failure, 94 responded to this question, of whom 24 (25.5%; 95% CI: 16.8–34.3) with chronic heart failure had been admitted to hospital in the previous 12 months. The highest rates of hospitalisation were in the oldest age group of 75+ years, with an age-specific rate of 28.4% (95% CI: 16.5–40.2), and among those with severe (NYHA class IV) chronic heart failure, with a class-specific rate of 54.5% (95% CI: 30.7–78.4).
Combining periods 2 and 3, 17 of 64 patients with chronic heart failure who were hospitalised (26.6%; 95% CI: 13.8–39.3) were known to have been discharged to a community-based heart failure management program.
Discussion
This study found that 2% of patients who attend general practice at least once in a year have diagnosed chronic heart failure, with an estimated population prevalence of 1.8%. This aligns with findings from another BEACH study of multimorbidity among 8989 patients, which reported a population prevalence of diagnosed chronic heart failure as 1.7% (95% CI: 1.4–2.1).13 However, our estimate of population prevalence is significantly higher than the estimate from patients’ self-report in the Australian Health Survey (1.4% in 2004–05, 1.3% in 2007–08, and 1.2% in 2011–12), although no confidence intervals are provided.14 However, self-report may underestimate the prevalence.14 Chronic heart failure was encountered in 3–4% of GP consultations.
Patients most commonly had mild heart failure, but a significant proportion (30%) had a more advanced stage of moderate to severe disease. Natriuretic peptide testing was used in less than one-quarter of patients with chronic heart failure. The medication burden was high, with patients taking a mean of 2.26 heart failure medication agents in addition to drugs for other comorbidities. Hospitalisation was common, but only about one in four patients were discharged from hospital to a community-based heart failure management program.
Globally, chronic heart failure is a common condition, often pre-dated by coronary artery disease, hypertension or other cardiovascular risk factors.15 It is the end result of considerable pathological insult to the heart, resulting in an inability to pump sufficient blood to meet the physiological needs of the body.16 Identifying patients at an earlier stage of disease may allow for more effective intervention, although this remains contentious. The clinical features of chronic heart failure, however, are non-specific, and this can make the diagnostic process challenging.17,18 For example, a symptom such as breathlessness is common and may be found in many other conditions.19
Natriuretic peptide testing is neither supported by Australian guidelines nor subsidised by Medicare, so represents a cost to the patient. Echocardiography, however, is both recommended and widely available.20 This study confirms that natriuretic peptide tests are used infrequently in primary care. Internationally, guidelines have increasingly recognised the role of natriuretic peptide testing to identify those patients who require referral for further investigation where echocardiography services are more limited.21,22
Hospitalisation can be traumatic for patients and costly to the healthcare system.4 Drugs that block the renin-angiotensin system and sympathetic drive have been proven to reduce hospitalisation and improve survival in patients with HFREF, but the benefit of these treatments in patients with heart failure with preserved ejection fraction has not been proven.23 New drugs, such as the neprilysin inhibitors, are also emerging that may change the way heart failure is managed in the future.24 The medication burden for now, however, remains high, both for patients to administer and for GPs to manage. More effective methods of managing patients with chronic heart failure within a community setting are needed.9
Implications for general practice
GPs have an essential role in the care of patients with chronic heart failure throughout their healthcare journey. The GP is crucial in recognising the initial symptoms of chronic heart failure and organising further tests or referral to a specialist, and subsequently initiating and optimising chronic heart failure treatments with proven prognostic benefit.20–22 GPs may also need to manage the acutely unwell patient and arrange hospitalisation.
This study shows that all stages of chronic heart failure are encountered by GPs. There is the potential for use of natriuretic peptide testing to be expanded, but this would depend on a review of guidelines and support for testing by Medicare. Medication schedules are complex and most drugs are still initiated by specialists. Patients with chronic heart failure often have several other comorbidities; optimising holistic care, including the avoidance of harmful polypharmacy, might be best achieved by the GP.
Despite pharmacological therapies, there is a high burden of hospital admissions and further measures to prevent them are necessary. Discharge to a community‑based heart failure management program only occurs in a small number of cases. In Australia most patients with heart failure are managed in conjunction with a cardiologist, but the general availability and configuration of hospital and community-based heart failure programs across the country is currently unknown. This configuration might be an important mechanism to explore for preventing future hospital admissions and optimising chronic heart failure management.
Authors
Clare J Taylor PhD, FRCGP, General Practitioner and National Institute for Health Research (NIHR) Academic Clinical Lecturer, Nuffield Department of Primary Care Health Sciences, University of Oxford, Oxford, UK. clare.taylor@phc.ox.ac.uk
Lisa Valenti BEc, MMedStat, Senior Analyst, Family Medicine Research Centre, University of Sydney, Parramatta, NSW
Helena Britt BA, PhD, Professor of Primary Care Research and Director, Family Medicine Research Centre, University of Sydney, Parramatta, NSW
Joan Henderson BAppSc (HIM) (Hons), PhD (Med), Senior Research Fellow, Family Medicine Research Centre, University of Sydney, Parramatta, NSW
Clare Bayram BAppSc (HIM) (Hons), PhD (Med), Research Fellow, Family Medicine Research Centre, University of Sydney, Parramatta, NSW
Graeme C Miller PhD, FRACGP, Medical Director, Family Medicine Research Centre, University of Sydney, Parramatta, NSW
FD Richard Hobbs MA, FRCGP, FRCP, FESC, FMedSci, Professor and Head of Department, Nuffield Department of Primary Care Health Sciences, University of Oxford, Oxford, UK
Competing interests: None.
Provenance and peer review: Not commissioned, externally peer reviewed.
Acknowledgements
We thank all the GP participants for their generosity.
The SAND sub-studies reported here were undertaken in collaboration with Novartis Pharmaceuticals Australia Pty Ltd and Seqirus (Australia) Pty Ltd (then bioCSL (Australia) Pty Ltd).
For 2010–11, 2013–14 and 2014–15, when these SAND sub-studies were undertaken, the BEACH program was funded by the Australian Government Department of Health and Ageing and Department of Veterans’ Affairs, AstraZeneca Pty Ltd (Australia), Merck, Sharp and Dohme (Australia) Pty Ltd, Pfizer Australia Pty Ltd, Sanofi-Aventis Australia Pty Ltd, Novartis Pharmaceuticals Australia Pty Ltd, GlaxoSmithKline Australia Pty Ltd, Seqirus (Australia) Pty Ltd (then bioCSL (Australia) Pty Ltd), Bayer Australia Ltd, AbbVie Pty Ltd.
The SAND sub-studies reported in this paper were conducted by the Family Medicine Research Centre and the funding bodies had no influence on the conduct of this research or preparation of this paper.