In just two decades, and with years of scientific advances worthy of multiple Nobel prizes, functional magnetic resonance imaging (fMRI) is now a mainstream neuroimaging modality in specialised centres worldwide. By 2001, about 700 MEDLINE articles related to fMRI had been published.1 At the time of writing, a replication of this search yielded more than 7300 articles.
Clinical fMRI is a technique that uses a standard MRI scanner to provide non-invasive functional mapping of brain activity during performance of dedicated motor, language, memory or visually based tasks called paradigms. fMRI has advanced our understanding of neuroanatomy in living, healthy individuals, as well as in those who have neurological diseases. The contributions of fMRI to the field of cognitive neuroscience have been vast; however, these are beyond the scope of this review. Currently, the principal clinical application of fMRI is in advanced neurosurgical planning for patients undergoing surgery for brain tumours or epilepsy.
In this article, we will introduce the basic physical principles of fMRI, details of its current clinical uses and limitations, and consider promising directions for future uses in clinical medicine, with a focus on implications for primary care.
The blood oxygen level–dependent effect
Neurons in the brain of a living human require a continuous supply of glucose from the blood. Although the brain comprises just 2% of total body weight, it consumes about 20% of glucose-derived energy.2 Neural activity directed at a specific task results in an almost immediate localised elevation of blood flow to the subservient brain regions to augment oxygen and glucose supply. The mechanism behind this process, known as ‘neurovascular coupling’, is not completely understood and sometimes mathematically represented as a haemodynamic response function (HRF).
The increase in neural activity results in an over-compensation of oxygen delivery, exceeding the amount that is required for the neurons, and locally increased oxygen extraction from the brain’s capillary bed. The change in oxygen extraction manifests as an increased ratio of oxygenated haemoglobin (oxyHb) to deoxygenated haemoglobin (deoxyHb) on either side of the capillary bed. This alteration is measurable using the blood oxygen level–dependent (BOLD) effect, the most frequently used physical means for generating fMRI data.3
While oxyHb is diamagnetic (weakly repelled from the field), deoxyHb is paramagnetic (weakly attracted to the field); accordingly, the amount and quality of MR signal derived from each is different. Specifically, the amount of deoxyHb magnetisation (‘susceptibility’) exceeds that of oxyHb by about 20%. This is manifested as increased T2-‘star’ (T2*)-weighted signal in highly oxygenated tissues, compared with deoxygenated tissues.4
By measuring the BOLD effect when the subject is at rest, and comparing this with the amount of signal generated during a carefully designed paradigm that is targeted at a particular functional process (eg motor, language-based, memory-based, visual), the precise functional localisation of nerve cells within the brain can be colour-mapped in healthy and diseased states. These functional maps can be co-registered with MRI anatomical data to provide detailed cortical activation maps for specialist interpretation. The majority of paradigms manipulate cognitive processes and are planned to occur over a period of a few seconds.
Additional information can be acquired from dedicated MRI sequences designed for patients with brain tumours and epilepsy, as well as white matter tract imaging (tractography) using diffusion tensor imaging (DTI). DTI is a complementary MRI technique that uses complex mathematics to accurately quantify directionality of water molecule movement along white matter tracts. For instance, these tracts may be disturbed by infiltrating or space-occupying tumours. This information can be colour-coded and represented with fMRI data on structural brain images.
Recent studies have also found that useful connectivity information may be derived from resting-state fMRI (intrinsic brain activity while doing nothing), particularly in patients without the capacity to follow the paradigms, and in less time, but with reduced test–retest reliability. Resting-state brain activity is reduced in patients with Alzheimer’s disease, compared with controls.5 However, this technique remains largely experimental at present.6,7
Functional neuroanatomy
The capacity to map blood flow variation related to brain activation during structured active tasks has led to a new understanding of neuroanatomy. Penfield’s seminal description of the somatotopic organisation of the precentral motor and postcentral sensory gyri of the brain in the 1950s was derived from direct cortical stimulation procedures. These maps have been partially reproduced using fMRI.8–10 To date, the only motor cortex structure with a consistent functional role is the hand–motor area of the precentral gyrus, first demonstrated in 1997 using fMRI.11 The primary auditory cortex is located at the transverse temporal gyrus of Heschl. The primary visual cortex is located in the striate cortex, mainly along the calcarine sulcus of the occipital lobe. There is far greater variability in brain cortex representations of other important functions, including language and memory.12,13 For example, fMRI has shown that Broca’s area (motor speech area), which is classically located at the left inferior frontal gyrus, may be bilateral or right-sided, possibly more so in females,14 and parts of its function may reside along the anterior insular cortex.15 Variation in language lateralisation in patients with epilepsy and those who are left-handed has been known for decades.16,17
Brain plasticity and injury recovery
The phenomenon of ‘cortical reorganisation’ or ‘neural plasticity’ is well recognised; most imaging studies showing this phenomenon are based on either positron emission tomography (PET) or fMRI. For example, displacement of Wernicke’s area (speech recognition area) from its expected location (posterior aspect of the left superior temporal region) to the right cerebral hemisphere in patients with brain tumours on the left has been shown with fMRI and validated with direct cortical stimulation.18 There is much interest in the role of fMRI in documenting patterns of recovery following stroke and other forms of brain injury or post-surgery,19,20 including fMRI evidence of plasticity following perinatal brain injury,21 in patients with arteriovenous malformations (AVMs),22 reorganisation of auditory brain areas after acoustic neuroma surgery23 and cochlear implant (using MRI and PET).24 New insights have also been gained into plasticity during normal ageing, and the relationship with age-related behavioural and memory impairment.25
Pre-surgical fMRI for brain tumours and AVM
Complete surgical resection of a brain tumour or AVM potentially endangers regions of functionally eloquent cortex or critical white matter pathways, posing a risk of a permanent neurological deficit to the patient. fMRI and tractography enable non-invasive visualisation of the anatomical relationship between functionally important brain regions and the tumour or AVM prior to surgery. This can help surgical teams plan how a radical resection might be safely achieved. This is particularly important when normal anatomical landmarks are moved or damaged by the lesion (Figures 1, 2).
By contrast, traditional methods of mapping the relationship between a tumour and nearby eloquent cortex are invasive. These currently include:
- direct intra-operative cortical stimulation during ‘awake craniotomy’
- subdural grid implantation with delayed, extra-operative stimulation mapping
- intra-operative recording of sensory-evoked potentials.
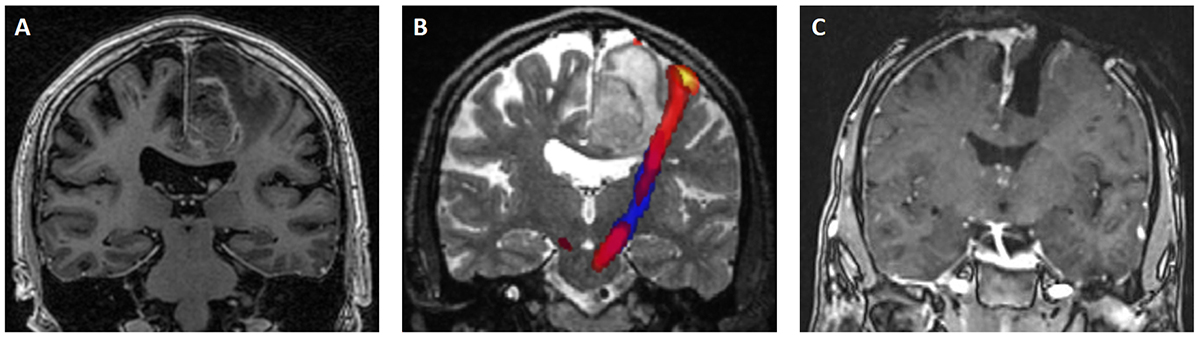
Figure 1. Example of combined fMRI and DTI informing a surgical approach on a man, 60 years of age, with known lung cancer who presented with seizures, followed by persisting right-sided weakness (right side of image is left side of patient)
A. Pre-operative coronal post-contrast T1 sequence shows a left parafalcine ring-enhancing tumour.
B. Combined fMRI/tractography demonstrated the CST related to the right-hand running lateral to the tumour. An appropriate neurosurgical approach was planned.
C. Intra-operative MRI shows complete lesion resection (note the open craniectomy, yet to be replaced). The lesion was a solitary metastasis from the lung primary. Partial improvement in right upper and lower limb strength followed.
DTI, diffusion tensor imaging; CST, corticospinal tract; fMRI, functional magnetic resonance imaging
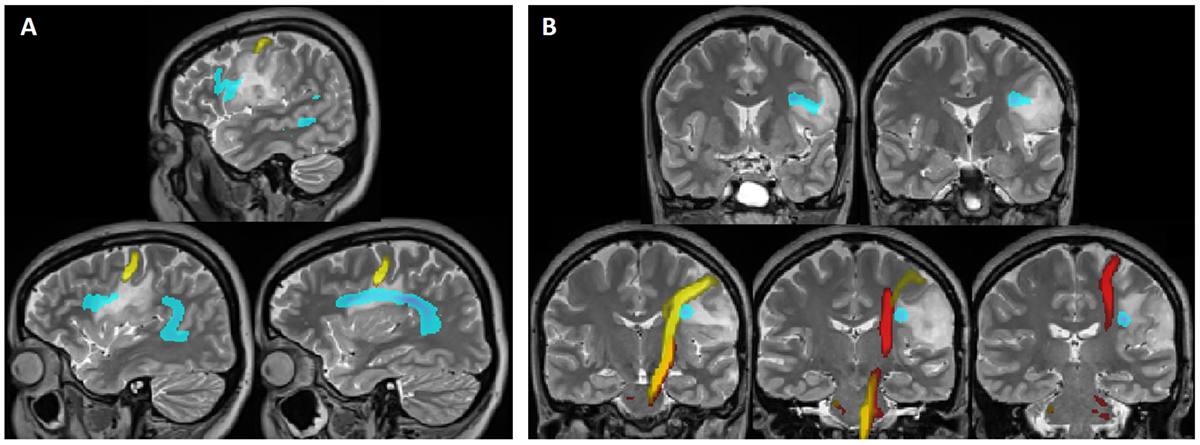
Figure 2. Example of combined fMRI and DTI aiding the surgical decision to defer surgery on a woman, 23 years of age, with a biopsy-proven left-sidedoligodendroglioma, stable over five years, but with recurrent seizures. fMRI was performed to assess whether surgical debulking of the tumour might improve seizure control. fMRI confirmed language dominance on the left (not shown)
Sagittal (A) and coronal (B) DTI images show the arcuate fasciculus (blue, white matter tract connecting Broca’s area and Wernicke’s area), CST for the right hand(red) and lips (yellow). Notice that the tumour encroaches upon the arcuate fasciculus and CST for lip function. The decision was made to recommend alteredanticonvulsant therapy rather than surgery for seizure control.
DTI, diffusion tensor imaging; CST, corticospinal tract; fMRI, functional magnetic resonance imaging
fMRI information can assist the surgical team to counsel the patient about the risk of functional impairment as a complication of the operation. fMRI also aids surgeons in their decision to perform an ‘awake craniotomy’ if the tumours are in close proximity to functionally critical regions. This may be required to validate fMRI data with direct intra-operative electric cortical stimulation techniques to determine appropriate resection margins.26
Pre-surgical fMRI for epilepsy
The aim of epilepsy surgery is to completely resect or disconnect the epileptogenic zone that is responsible for seizures in patients whose condition is resistant to medical therapy. Most frequently, this involves the temporal lobe.
fMRI has become a routine element in assessment prior to epilepsy surgery, together with seizure semiology analysis, electroencephalography (EEG), structural MRI and functional/neuropsychiatric assessments. Understandably, surgical units in adult and paediatric epilepsy centres involve large multidisciplinary teams (Figure 3).
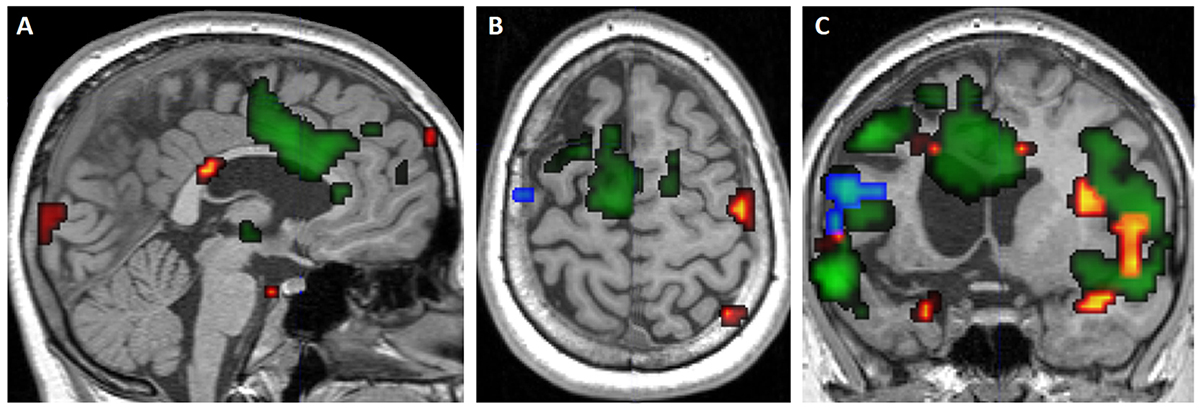
Figure 3. Example of cortical reorganisation (‘plasticity’) of language functions in a child, 12 years of age, suffering from daily, drug-resistant seizures secondaryto Rasmussen’s encephalitis (left side of image is left side of patient)
Images are sagittal (A, D), axial (B, E) and coronal (C, F) sections.
Serial fMRI scans commencing in 2008 at 12 years of age demonstrated initial left-sided language dominance (blue), becoming bilateralised on the scan performed in 2009 at 13 years of age (green) and 2010 at 14 years of age (red) scans. This provided important data for a subsequent hemispherotomy (F).The patient is now aged 18 years, seizure free (maintained on medication), and with preserved language function on the right (D–F, yellow).
At present, the primary contribution of fMRI in epilepsy surgery is the identification of language-processing regions and hemispheric dominance, to predict and minimise language deficits. On the basis of strong research evidence, fMRI has effectively replaced the invasive ‘Wada’ test (awake intra-arterial catheter injection of sodium amobarbital or propofol selectively into one hemisphere), which was previously used for this purpose.27
Several fMRI studies have found reorganisation of the language centre in young patients with epilepsy due to frontal and temporal brain lesions.28,29 Interestingly, higher seizure activity may drive this process.30 Paediatric fMRI targeting language lateralisation can be performed in some specialised centres, and may also have a role in predicting memory impairment (Figure 3).31 Several centres are investigating the acquisition of simultaneous fMRI and EEG data in identifying epileptogenic foci.27,32
Limitations
There are numerous limitations to the clinical application of fMRI data, which require specific attention. The amount of BOLD signal generated by haemodynamic responses to neural activity is extremely small. Therefore, strategies to maximise useful signal (in this case, task-related BOLD signal) from noise (eg scanner and associated hardware, motion artefacts, non–task related neural activity, individual performance of various task paradigms) are crucial.
The absence of activation, for example, following mechanical compression of vessels by a tumour, does not imply a lack of cortical importance or ‘eloquence’. Conversely, the presence of activation does not always correspond to functional significance.
fMRI is vulnerable to all the artefacts that apply to standard, anatomical MRI, including motion artifact, effects of prior surgery, and the relative contraindications to patients with internal metalware and claustrophobia.
The consistency of the within-subject and repeatability of between-subject variability in the BOLD haemodynamic response have been shown to be high, but require statistical thresholding.33 Variability of human neuroanatomy and the potential for cortical reorganisation in disease states are further challenges to the interpretation of fMRI maps.
To mitigate these limitations, fMRI must be performed in dedicated neuroimaging centres by an expert team of experienced physicists, neuroscientists, radiographers and radiologists. fMRI information should be considered alongside additional clinical data and, where necessary, direct measures of electrical stimulation of the cortex to surgically validate the fMRI findings.
Future clinical uses of fMRI
Research applications of fMRI and PET are extending the role of imaging in the early detection of neurodegenerative diseases, particularly Alzheimer’s disease, differentiation of dementia subtypes, and detection of pre-clinical Parkinson’s disease.34
Promising research directions in fMRI include the use of meta-analysis and machine-learning technology to combine results of large numbers of studies to create activation-likelihood maps, thereby increasing statistical power and addressing inference errors.35
The Human Connectome Project is acquiring large volumes of functional imaging data to correlate the detailed anatomy of the white matter tracts (now seen in detail with DTI) with genetic and behavioural substudies.36 fMRI has been used to study drug mechanisms, efficacy and development of new drugs, particularly in psychiatry.37
At research facilities, ultra–high field human fMRI now achieves approximately 1 mm3 resolution at 7T. The anticipated installation of the world’s strongest 11.75T human MRI this year in Saclay, France, promises unprecedented submillimetre resolution, at a field strength 250,000 times that of Earth, redefining the boundaries of human imaging.
Conclusions
GPs can expect to be exposed to increasing numbers of patients requiring advanced imaging techniques, including fMRI and DTI, for optimal state-of-the-art treatment of brain tumours and epilepsy.
Our understanding of functional neuroanatomy, neural plasticity, pathophysiology and drug effects has expanded greatly using fMRI, and these areas are still fields of intense research activity. DTI is performed with fMRI to enable localisation of white matter tracts that may be affected by the brain disease under investigation. At present, the clinical application of fMRI is principally limited to pre-surgical assessment of patients with brain tumours and epilepsy. These patients are referred by neurologists and neurosurgeons to specially equipped neuroimaging centres with expertise in acquiring and interpreting these images. However, new applications in the fields of neurodegenerative disorders, brain injury and stroke rehabilitation are likely in the near future.
With this knowledge, including the limitations of fMRI, primary care doctors are better equipped to deal with patients of all ages, and their families, in explaining the role of fMRI in clinical management.
Key points
- fMRI is a non-invasive imaging modality that enables measurement of transient haemodynamic changes in the brain during carefully designed active tasks (paradigms) usually referring to motor, language, memory or visual functions.
- fMRI has an established role in the pre-surgical assessment of adult and paediatric patients being considered for brain tumour or epilepsy surgery, where the expected resection margin is close to regions of eloquent cortex.
- fMRI is performed within most major neurology and neurosurgery centres. Referral is currently limited to neurologists and neurosurgeons, and usually following case discussion by multidisciplinary epilepsy and brain tumour specialist teams.
- Awareness of the current and potential future applications of fMRI should assist GPs in informing and counselling a gradually increasing number of patients undergoing this examination.
Authors
Christen D Barras MBBS, BMedSci, MMed, PhD, DipSurgAnat, DipOMS, FRANZCR, Neuroradiology Fellow, Lysholm Department of Neuroradiology, National Hospital for Neurology and Neurosurgery, London, UK; Senior Lecturer, Department of Radiology, University of Melbourne, Vic. christenbarras@gmail.com
Hamed Asadi MD, PhD, FRANZCR, Consultant Interventional Neuroradiologist, Neuroradiology and Neurointerventional Service, Department of Radiology, Beaumont Hospital, Dublin, Ireland; Senior Lecturer, Deakin University, Vic
Torsten Baldeweg MD, Professor of Developmental Cognitive Neuroscience, University College London, UK; UCL Great Ormond Street Institute of Child Health, Developmental Neurosciences Programme, Cognitive Neuroscience and Neuropsychiatry Section, London, UK
Laura Mancini PhD, MRI Clinical Scientist, Lysholm Department of Neuroradiology, National Hospital for Neurology and Neurosurgery, London, UK
Tarek A Yousry Dr med Habil, FRCR, Professor of Neuroradiology, Head of the Neuroradiological Academic Unit, Head of the Division of Neuroradiology and Neurophysics, University College London, Institute of Neurology, London, UK; Head of the Lysholm Department of Neuroradiology, National Hospital for Neurology and Neurosurgery, UCLH Foundation Trust, London, UK
Sotirios Bisdas MD, PhD, MSc (Advanced Oncology), FESHNR, Professor, Consultant Neuroradiologist, Lysholm Department of Neuroradiology, National Hospital for Neurology and Neurosurgery, London, UK
Competing interests: None.
Provenance and peer review: Commissioned, externally peer reviewed.