Recent changes to the diagnostic threshold and process of universal oral glucose tolerance testing may increase the number of women diagnosed with gestational diabetes mellitus (GDM) by up to 50%.1 Allergic reactions to insulin administration are uncommon in clinical practice. The risk of allergy during pregnancy is even lower because of the immunosuppressive state of pregnancy.2 Fewer than 20 cases of insulin allergy worldwide have previously been reported during pregnancy.3 Cases of three patients who presented with localised reactions at subcutaneous injection sites within one week of treatment with insulin detemir for GDM are discussed.
Case 1
A primigravida of Chinese descent, 32 years of age, was commenced on 20 units of insulin detemir nocte at 34 weeks gestation because of elevated fasting blood glucose levels in the setting of GDM. Approximately one week after commencing insulin therapy, she noted pruritic, painful, erythematous reactions developing at the injection site from the previous night (Figure 1). The reactions enlarged over the subsequent two to three days, then gradually resolved over four to five days. There was a satisfactory glycaemic response to insulin administration. There were no further reactions at injection sites on changing to insulin glargine.
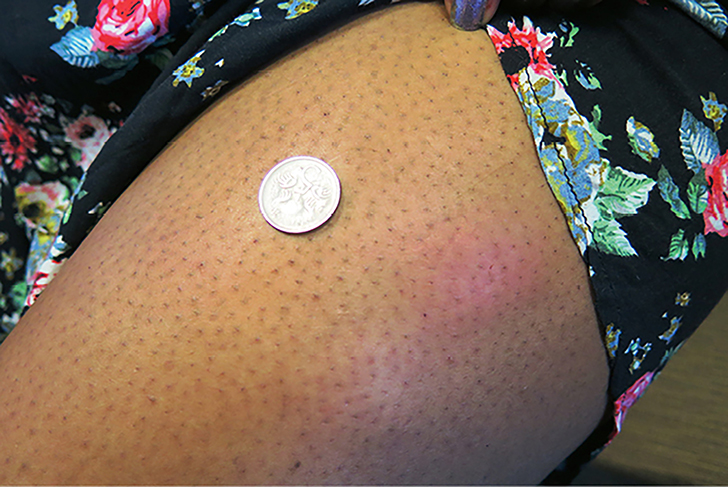 |
| Figure 1. Insulin reactions anterolateral thigh (Case 1) |
Case 2
A primigravida of Indian descent with GDM, 26 years of age, was commenced on insulin detemir at 28 weeks gestation. Approximately two weeks after commencing insulin therapy, she noticed generalised pruritus as well as tender, painful, erythematous nodules that appeared approximately eight hours after each insulin injection (Figure 2). The nodules resolved spontaneously over a period of four to five days. The patient was changed to insulin glargine without further issue.
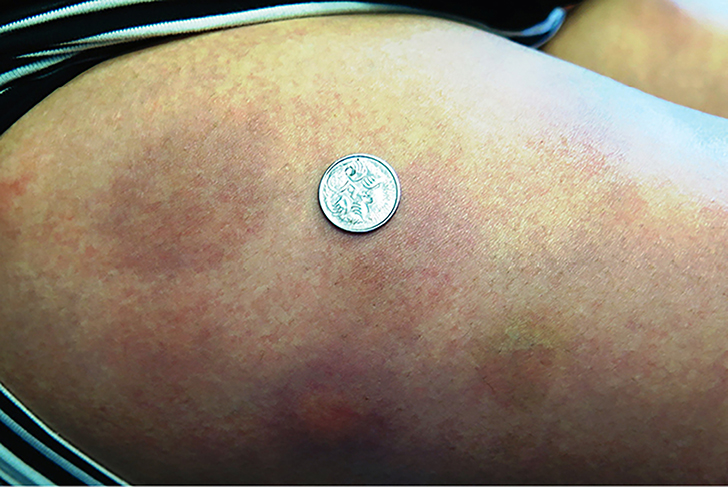 |
| Figure 2. Insulin reactions anterolateral thigh (Case 2) |
Case 3
A gravida 2 para 1 of Chinese descent, 39 years of age, noted the development of pruritic tender erythematous nodules 24 hours after injecting insulin in her thighs, having commenced insulin detemir for GDM a fortnight earlier. The dose was gradually titrated from 8 units to 26 units nocte. She was changed to insulin glargine with no further reaction.
None of the patients manifested dermatographism. Discussion with colleagues revealed that six other women with GDM who had similar reactions to insulin detemir administration were seen at the same institution over the preceding six months.
Discussion
Neutral protamine Hagedorn insulin is the basal insulin of choice for women with GDM.4 Insulin detemir and glargine do not have Pharmaceutical Benefits Scheme (PBS) listing for use in GDM, although these insulin analogues are safe treatment options during pregnancy.4
Immediate type I or immunoglobulin E (IgE)-mediated allergic reactions are the most frequently reported reactions to insulin administration; more than 300 cases worldwide have been reported since 1950.5 The incidence of allergic episodes has decreased significantly with the advent of insulin analogues.6 Anaphylaxis may occur, and at least one death has been attributed to insulin allergy.6 Type III immune complex-type (localised Arthus reaction or generalised serum sickness) reactions and delayed type IV hypersensitivity reactions following insulin administration may also occur. Co-administration of angiotensin converting enzyme inhibitors and HMG Co-A reductase inhibitors may precipitate local reactions to insulin.7
Reactions may not be due to insulin itself but to non-insulin additives in preparations including myristic acid, mannitol, protamine sulphate, zinc, glycerol, phenol and metacresol. Type I hypersensitivity reactions to natural rubber latex in the insulin vial membrane should also be considered. The management of allergic reactions to insulin administration may be difficult, especially in patients with type 1 diabetes mellitus. Strategies used have included:8
- desensitisation using continuous subcutaneous infusion of short-acting insulin via insulin pump
- inhaled insulin
- trials of alternative long-acting insulin preparations
- administration of oral and subcutaneous antihistamine
- immunosuppressive therapy
- pancreas transplantation.
A recent case report described the successful use of insulin degludec combined with liraglutide in a woman with poorly controlled type 2 diabetes mellitus who was allergic to all other insulin formulations.9
Twenty-three previous cases of allergic reaction following the administration of insulin detemir to patients who were not pregnant have been published. Six patients were identified over a period of six months by a group in France, and three patients with insulin allergy presented over a period of a few months in the UK in the same year.10,11 Six of the 52 pregnant women who received insulin detemir as part of a clinical trial over an 18-month period in New York ceased the medication as a result of unanticipated allergic rash.12 This clustering of reactions within geographical localities in discrete time periods raises the possibility of an allergenic contaminant within batches of insulin.
The cases presented in this article may represent an inflammatory reaction to an excipient contaminating the insulin used, or a localised Arthus reaction to insulin. A severe serum sickness-like type III reaction to insulin detemir has been reported previously.13 Samples of insulin detemir used by the patients reported in this manuscript have been sent to the manufacturer for analysis.
Key points
- Insulin allergy is uncommon but may be life-threatening in rare cases.
- Insulin allergy may be due to excipients in the preparation rather than the insulin itself.
- Altering the type of insulin may result in resolution of allergic reactions.
Authors
Adam Morton FRACP, Senior Staff Specialist, Endocrinology and Obstetric Medicine, University of Queensland and Mater Health Services, South Brisbane, Qld. Adam.morton@mater.org.au
Josephine Laurie FRACP, Obstetric Medicine, University of Queensland and Mater Health Services, South Brisbane, Qld
Competing interests: None.
Provenance and peer review: Not commissioned, externally peer reviewed.