Chronic strongyloidiasis in humans is caused by the remarkably persistent roundworm Strongyloides stercoralis, distinguished by its unique autoinfective lifecycle. General practitioners (GPs) have an important role in diagnosing and treating chronic strongyloidiasis to prevent cases of fatal hyperinfection. Unless strongyloidiasis is deliberately considered, the diagnosis is unlikely to be made.
Epidemiology
An estimated 370 million people worldwide are infected with S. stercoralis.1 Strongyloidiasis is endemic in tropical and subtropical regions of the world where warmth, moisture and poor sanitation favour its spread. A prevalence greater than 5% is considered hyperendemic.2 Some remote Aboriginal and Torres Strait Islander communities in Australia have had prevalences up to 60%.3 In Australia, strongyloidiasis should be considered in residents of endemic areas, immigrants (including older patients from southern Europe), refugees, war veterans (World War II and Vietnam War), and workers and travellers returning from endemic areas with ‘a souvenir you don’t want to bring home’.4–7
Who is at risk?
Although history is important to identify those at risk of strongyloidiasis, the initial exposure to infective larvae may have occurred decades earlier. Doctors should consider infection with S. stercoralis in all patients who are to receive immunosuppressive therapy (especially corticosteroids and chemotherapy), as this can cause the hyperinfective syndrome.
Patients who are immunocompromised, such as those with diabetes, systemic lupus erythematosus (SLE), human T-cell lymphotropic virus type 1, or organ transplant recipients, as well as patients who are malnourished and infected, are at risk of dissemination.8 Aboriginal and Torres Strait Islander patients from rural and remote areas in Australia should not be given immunosuppressive treatment without being tested or treated prophylactically for strongyloidiasis.9
Life cycle
The traditional mode of S. stercoralis infection is penetration of the skin by microscopic, infective filariform larvae. The larvae are then carried in the bloodstream to the right side of the heart. They exit the pulmonary capillaries, enter the alveolar spaces in the lungs, ascend the bronchial tree and are swallowed by the host. In the small intestine, the larvae mature into adult female worms (2–3 mm long) and penetrate the mucosa of the proximal small intestine, where they can lay up to 40 eggs a day. This occurs 17–28 days after the initial infection. The eggs hatch in the intestinal mucosa to release rhabditiform larvae that migrate to the lumen of the intestine.2
The life cycle of S. stercoralis has three unusual features that make its eradication difficult:
- Autoinfective larvae: these can penetrate the colonic wall or perianal skin, and enter the body to repeat the migration that establishes ongoing internal reinfection. This autoinfective cycle allows strongyloidiasis to persist for decades after the host has left an endemic area. Hyperinfection or disseminated strongyloidiasis occurs because large numbers of parasitic females develop in the small intestine and thousands of autoinfective larvae migrate through the organs.2 Prescribing immunosuppressants, especially corticosteroids, to undiagnosed patients who are infected is a common precipitant for disseminated strongyloidiasis because it stimulates the autoinfective cycle.2,8,10
- Parthenogenesis: the ability of the parasitic female to reproduce without a male contributes to the challenges of successful treatment in humans. One remaining female parasite can result in recrudescence of infection.
- External phase of life cycle and free-living adults: S. stercoralis has the ability to produce one generation of short-lived, free-living adult male and female worms in the external environment. Only one generation of free-living adults are produced by S. stercoralis and they die within 10 days.2,11,12 The infective larvae can survive in the soil for several weeks if the environment is moist and temperature is suitable (23–28°C).11
Clinical presentations
The autoinfective larvae of S. stercoralis can invade any organ of the body, including the central nervous system, through random migration.2,13–15 The symptoms of chronic strongyloidiasis may be protean, non-specific and intermittent, making the underlying diagnosis elusive. Signs and symptoms vary with the location and number of worms, and whether the autoinfective larvae have carried bacteria to extraintestinal locations. The following clinical presentations may alert the clinician to consider the diagnosis of strongyloidiasis:
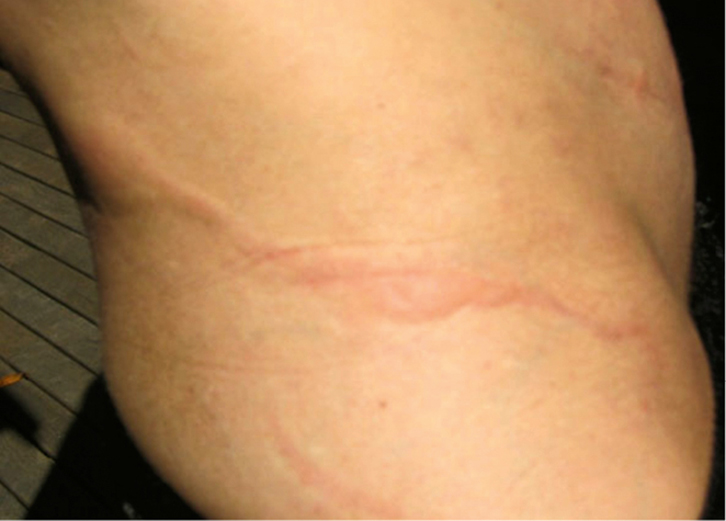 |
Figure 1. Larva currens on the trunk of female patient from northern New South Wales
These erythematous, serpiginous lesions appear and move rapidly (2–10 cm per hour), and are the pathognomonic sign of strongyloidiasis. Source: W. Page |
- Skin: larva currens is the only pathognomonic sign in patients with S. stercoralis infection. Erythematous serpiginous lesions appear and move rapidly (2–10 cm per hour). This is more commonly seen on the trunk and buttocks (Figure 1). Other signs include pruritus, recurrent urticaria or a ‘rash that comes and goes’.2,4,14 A ‘rapidly progressive purpuric petechial eruption with a reticulated pattern’ has been described in disseminated strongyloidiasis.16
- Respiratory: pulmonary strongyloidiasis manifestations include dyspnoea, bronchospasm, haemoptysis, bronchopneumonia, pleural effusion, lung abscess and interlobular septal fibrosis.14,17 There are reported cases of immigrants in Australia with cavitary lesions on chest X-ray that mimicked tuberculosis but resolved after treatment with anthelmintics.18
- Gastrointestinal: manifestations may include diarrhoea, hypokalaemia, protein-losing enteropathy, malnutrition, wasting, epigastric pain and tenderness that simulates peptic ulcers, subacute obstruction or segmental ileus, paralytic ileus, ulcerative enteritis with intestinal perforation and peritonitis, melaena, and haematochezia.14,15,19
- Genitourinary: manifestations may include nephrotic syndrome and renal abscess.15,20
- Hepatic: manifestations may include hepatomegaly and hepatic abscess.15,21
- Cardiac: manifestations may include pericardial effusion.22
- Haematological: eosinophilia is present in 10–70% of chronic strongyloidiasis cases, but is less prevalent in disseminated strongyloidiasis.
- Central nervous system (CNS): manifestations may include bacterial meningitis and abnormal CNS signs.2,13,23
- Sepsis: Gram-negative septicaemia or sepsis in any organ may occur due to enteric bacteria.15,24
- Multiple end-organ failure: this is an outcome of disseminated strongyloidiasis with a poor prognosis despite treatment.2,14,24
Early diagnosis improves outcome
Prevention of high morbidity and mortality from strongyloidiasis is dependent on clinicians’ awareness. Most cases of disseminated strongyloidiasis are diagnosed at autopsy or in the late disseminated phase, when millions of parasites are in the body, which means they are more easily identified in body fluids. Unfortunately, this is too late for the majority, who will die from this disease. All individuals who are infected, including those who are asymptomatic, should be treated because hyperinfection is unpredictable and potentially fatal.14,15
GPs have an important role in the diagnosis, treatment and follow-up of patients with chronic strongyloidiasis by:
- identifying patients at risk (who may be asymptomatic)
- considering strongyloidiasis as a differential diagnosis or underlying cause for non-specific clinical manifestations as outlined above. Gram-negative sepsis should be a prompt to consider strongyloidiasis as an underlying factor11,23,24
- screening patients who are at risk and advising prophylaxis prior to immunosuppressant therapy8,9,25
- considering strongyloidiasis in patients with unexpected clinical deterioration on immunosuppressive therapy (eg asthma,26 malignancy).
Diagnosis
The usefulness and sensitivity of diagnostic testing is affected by the phase of strongyloidiasis – acute, chronic or disseminated. The different phases, likely larval load per millilitre of faeces, and immune responses are described in Table 1. Faecal testing has high sensitivity in acute and disseminated strongyloidiasis, whereas serology is most useful in diagnosing chronic strongyloidiasis in patients who are immunocompetent.
Table 1. Interpreting faecal and serology diagnostic tests for phases of strongyloidiasis2,11,27,32
|
| Phase | Larvae
(mL of faeces) | Strongyloides serum IgG antibody levels | Immune response and parasite activity |
|---|
Acute –
Initial infection may have significant gastrointestinal and non-specific symptoms |
0 to >1000
Standard usually positive |
Negative, equivocal or positive |
Large number of parasitic females in intestine, then larval output slows as immune response and eosinophils increases. ‘Window period’ before specific IgG antibody levels become raised |
Chronic –
Non-specific, intermittent symptoms over decades.
A control study found up to 70% of patients with intermittent symptoms2 |
0–400
Standard faecal tests usually negative
Special tests using multiple specimens required |
Positive or equivocal |
Immune response keeps the number of parasites lower.
Higher IgG titre is indicative of appropriate immune response rather than high parasite numbers.
Eosinophilia intermittent: 10–70% |
Hyperinfective/disseminated strongyloidiasis –
Exponential increase in parasites throughout body. Increased rates of sepsis and multiple end-organ failure.
High fatality rate |
400 to >1000
Standard faecal test usually positive
Sputum and body fluids likely to be positive for autoinfective larvae |
Positive,
equivocal or negative |
Immune response decreases and accelerated autoinfection:
Autoinfective cycle is ‘out of control’. Patients who are immunosuppressed, immunocompromised and malnourished have reduced ability to generate adequate immune response. Eosinophilia is less common in hyperinfection |
|
IgG, immunoglobulin G
|
Faecal examinations
Patients with chronic strongyloidiasis may have multiple negative test results on faecal specimens.27 Faecal testing has a higher sensitivity in acute and disseminated phases because of the high numbers of larvae in faeces (see Table 1). Faecal testing has been the mainstay of testing for ova, cysts and parasites. The presence of live larvae, rather than eggs, in fresh faecal specimens is a feature of S. stercoralis. Cool storage, transportation and delays before microscopic examination in a laboratory reduce the chance of a positive diagnosis. Agar plate testing relies on live larvae to track across the plate.28 More recent molecular diagnostics hold hope for improved sensitivity.8 Including ‘strongyloides’ as a possible diagnosis on the clinical indication section would assist laboratory scientists in selecting the most appropriate faecal diagnostic test.
Serology
Strongyloides serology using the strongyloides immunoglobulin G (IgG) enzyme-linked immunosorbent assay (ELISA) has a high sensitivity and specificity for chronic strongyloidiasis.2,8,15,27 Strongyloides-specific IgG relies on measuring the body’s immune response to the presence of S. stercoralis. The immune response varies for different phases of strongyloidiasis and is described in Table 1. Sensitivity may be reduced in acute strongyloidiasis and hyperinfection.27 False negatives can occur when new cases have not yet seroconverted. Also, patients who are severely immunocompromised may be unable to generate adequate IgG or a raised eosinophil count. Strongyloides-specific IgG serology decreases with effective treatment and is useful in monitoring eradication.29–32
Treatment
The goal of therapy is eradication of all S. stercoralis parasites. One remaining parasite has the ability to replicate and cause a recrudescence of infection.2
Ivermectin is currently the most effective therapy for the eradication of
S. stercoralis. Albendazole can be used when ivermectin is not suitable (eg concurrent loiasis, which is caused by the parasitic worm Loa loa).8 However, although the intensity of infection will be reduced with albendazole, the cure rate is lower.4,32–34 If immunosuppressants are required for a patient at risk of strongyloidiasis, prophylactic treatment is warranted. Hyperinfection is life threatening and requires specialist advice.8,35
Table 2. Therapeutic Guidelines for strongyloidiasis35,36
|
To treat strongyloidiasis (Strongyloides stercoralis) in immunocompetent patients, use:
- Ivermectin (adults and children weighing 15 kg or more) – 200 µg/kg orally with fatty food for one day and repeat after 7–14 days (Category B3 in pregnancy).
OR
- Albendazole – 400 mg (200 mg for children weighing 10 kg or less) orally with fatty food; 12-hourly for three days and repeat after 7–14 days (Category D in pregnancy).
To treat immunocompromised patients, use:
- Ivermectin (adults and children weighing 15 kg or more) – 200 µg/kg orally with fatty food on days 1, 2, 15, 16. Immunocompromised patients requiring immunosuppression are at high risk of disseminated strongyloidiasis (hyperinfection) and may require longer courses of therapy. Seek expert advice.
Prophylaxis prior to immunosuppression for patients at risk:
- If strongyloides serology is positive, treat with ivermectin 200 mcg/kg orally with fatty food, once weekly for two doses.
- If strongyloides serology is negative (from remote Aboriginal and Torres Strait Islander communities), use ivermectin 200 mcg/kg orally with fatty food as a single dose.
- Ongoing primary prophylaxis once every three months is recommended for patients requiring significant immunosuppression and returning to endemic communities.
Current for March 2015. Refer to Therapeutic Guidelines.
|
Follow-up of chronic strongyloidiasis
Best practice involves follow-up to ensure eradication. Strongyloides serology is useful to monitor the effectiveness of therapy. A negative result after approximately six months indicates successful therapy. Repeated courses of treatment and further follow-up may be required. Eosinophilia may be an indication of recrudescence. Current criteria for cure require negative serology, negative faeces and no symptoms, although an element of uncertainty as to whether the last parasite has been eradicated will remain.
Strongyloides control programs in endemic communities
Control strategies in endemic communities are now possible with improved diagnostic testing and effective treatment regimens.12 Some health services in endemic communities have incorporated strongyloides serology into biennial adult health checks to ensure better management of patients with chronic strongyloidiasis.27,32
Primary prevention
Transmission can be decreased by:
- improving access to sanitation facilities and environmental health education
- eradicating S. stercoralis infection in all people in a community to eliminate the source of the infective larvae. Population health strategies need implementation in endemic communities.12,27
Conclusion
Strongyloidiasis is a potentially fatal disease that is rarely seen in mainstream Australian communities where access to First World standards of sanitation facilities prevents transmission. It is a condition that is preventable and treatable if diagnosed early. GPs must be vigilant to ensure that cases are detected and the risks of fatal, disseminated disease reduced.
Key points
- Think of strongyloidiasis before starting a patient on immunosuppressant therapy, including corticosteroids, especially those from endemic areas or with unexplained eosinophilia.
- All patients who are infected, including those who are asymptomatic, should be treated.
- Eradication to the last worm is the goal of therapy.
Authors
Wendy Page MBBS, FRACGP, FACRRM, MPH&TM, PhD candidate, Miwatj Health Aboriginal Corporation, Nhulunbuy, NT. wendy.page@my.jcu.edu.au
Rick Speare PhD, MBBS (Hons), BVs (Hons), FAFPHM, FACTM, MACVS, Emeritus Professor, Public Health and Tropical Medicine, James Cook University, Townsville, QLD
Competing interests: None.
Provenance and peer review: Not commissioned, externally peer reviewed.