In contrast to women who experience a sudden drop in oestradiol levels around the time that menses ceases, the age-related drop in testosterone in men is more gradual at 0.5–2.0% per year from early adulthood onwards. As levels of sex hormone binding globulin (SHBG) rise with age by 1–2% per year, the decline in free testosterone level is greater and is 2–3% per year. Most older men have testosterone levels within the reference range and there is recent evidence from Australia that healthy ageing alone may not be associated with marked decreases in testosterone levels.5 However, the age-dependent decrease in testosterone levels is accelerated by accumulation of comorbidities, especially obesity.6 Indeed, a normal testosterone level can be considered to be a sensitive biomarker of good health.
Diagnosis of hypogonadism
The diagnosis of androgen deficiency should be made only in men with consistent symptoms and signs, and unequivocally and repeatedly low serum testosterone levels.1 In practice this diagnosis is often difficult, especially in older obese men with chronic disease, as symptoms and signs are often non-specific and because there is no evidence-based, universally agreed pathological testosterone cut-off level.1,7
Clinical diagnosis
Given that hypogonadism is primarily a clinical diagnosis supported by consistent biochemical findings, a thorough history and examination are essential. Clinical assessment should be focused on eliciting symptoms and signs of androgen deficiency and on identifying clues to the underlying aetiology (Table 18). Signs and symptoms include incomplete or delayed sexual development (if hypogonadism occurs before or during puberty), reduced libido, decreased spontaneous erections, breast discomfort, loss of body hair, reduced shaving, very small (especially <5 ml) or shrinking testes, infertility, height loss, low trauma fracture, low bone mineral density, hot flushes. It should be kept in mind that clinical features can be non-specific and related to other medical conditions. In men aged over 50 years, digital rectal examination must be performed to exclude palpable prostate pathology before commencing testosterone replacement therapy. In addition, although controversial in the general population, regular testing for prostate-specific antigen (PSA) is recommended during testosterone therapy and a baseline PSA of >4 ng/ml without urological evaluation is a contraindication to testosterone treatment.1
Table 1. Causes of androgen deficiency8
| Partial/transient | Primary (elevated FSH/LH) | Secondary (low/normal FSH/LH) |
|---|
Acute illness
Chronic disease
Androgen deprivation therapy (GnRH agonists)
Anabolic steroids |
ACQUIRED
Testicular damage
- Trauma
- Orchitis
- CTX/RTX/toxins
Drugs
- Spironolactone
- Ketoconazol
|
STRUCTURAL
Tumour
Surgery
Radiation
Trauma
Infiltration
- Iron overload
- Sarcoid
- Histiocytosis
|
CONGENITAL
Klinefelter syndrome
Cryptorchidism
Mutations in androgen biosynthesis enzymes
LH/FSH-receptor mutations
Myotonic dystrophy |
GENETIC
Kallmann syndrome
'Idiopathic' HH
LH/FSH beta subunit mutations |
FUNCTIONAL
Hyperprolactinaemia
Morbid obesity
Cushing’s syndrome
Alcohol excess* |
COPD, chronic obstructive pulmonary disease; CTX, chemotherapy; ESRF, end stage renal disease; RTX, radiotherapy; HH, hypogonadotropic hypogonadism; T2DM, type 2 diabetes mellitus
*Alcohol excess typically causes mixed (combined primary and secondary) hypogonadism. |
Biochemical diagnosis
Of the total circulating testosterone, 60% is tightly bound to SHBG, 38% is loosely bound to albumin and only 2% is free. Bioavailable testosterone refers to albumin-bound and free testosterone. Total testosterone is the mainstay of biochemical diagnosis of androgen deficiency.
The initial diagnostic test in suspected androgen deficiency is measurement of fasting morning total testosterone in men with consistent symptoms and signs. Patients should be well or medically stable, without acute decompensation of any underlying comorbidity. As testosterone release is diurnal, with the highest levels in the early morning, blood samples should be taken close to 8 am. Food intake can reduce total testosterone acutely by as much as 25%, explaining the importance of a fasting blood sample. There is marked variability in testosterone levels not only between individuals but also within an individual. Therefore, repeated measurements are necessary to confirm a low testosterone level and a diagnosis of hypogonadism should never be based on a single testosterone level.
There is no general agreement on the acceptable normal range of testosterone. Reference intervals also vary between laboratories because of differences in assay methods and/or reference population of men. In practice, if the total testosterone level is 12 nmol/L or above, the patient is usually eugonadal and further testing is generally not required. If the total testosterone level in a man with consistent symptoms and signs is less than 12 nmol/L, a repeat measurement of fasting morning testosterone level is suggested. Levels of 8–12 nmol/L may be considered borderline and <8 nmol/L low. If a testosterone level is borderline, requesting measurement of free testosterone may be helpful because the levels of total testosterone can be affected by alterations in levels of SHBG and albumin. Men with obesity and diabetes commonly have a low SHBG and here a normal free testosterone can be reassuring that such men are not, in fact, hypogonadal.
In general, a repeatedly low testosterone level is more indicative of hypogonadism in younger, healthier and leaner men but more difficult to interpret in older obese men with chronic disease and non-specific symptoms.6,7
Aetiology of hypogonadism
Once the low testosterone value has been confirmed on repeated morning measurements in patients with consistent symptoms and signs, luteinizing hormone (LH) and follicle stimulating hormone (FSH) values should be obtained to further distinguish between primary or secondary hypogonadism (Table 1). Elevated LH and FSH values indicate primary (testicular) hypogonadism, whereas low or importantly even inappropriately ‘normal’ LH and FSH values may indicate secondary (pituitary–hypothalamic) hypogonadism. Combined primary and secondary hypogonadism have variable gonadotropin levels, depending on whether primary or secondary hypogonadism predominates.
Primary hypogonadism
It is important to ask the patient about the age of onset of his problems, about congenital defects such as cryptorchidism, pubertal development, fertility, previous testicular trauma or infection, radiotherapy, chemotherapy, use of medications that inhibit androgen biosynthesis, and medical conditions that can cause both primary and secondary hypogonadism. A karyotype analysis should be obtained in men with primary testicular failure to exclude Klinefelter’s syndrome (47XXY), which occurs in about 1 in 500 men.3
Secondary hypogonadism
Organic congenital causes of secondary hypogonadism are rare and include Kallmann syndrome, GnRH receptor mutation and deficiency, or genetic mutations associated with other pituitary hormone deficiencies. These congenital disorders are usually diagnosed in childhood or adolescence when patients present with pubertal delay. Acquired causes of secondary hypogonadism are summarised in Table 1. Androgen deprivation therapy given to men with prostate cancer is an increasingly important cause of severe androgen deficiency.
Many chronic diseases are associated with low testosterone levels via suppression of gonadotropin production. Testosterone levels are commonly lowered in men with metabolic syndrome, type 2 diabetes mellitus, obesity, depression, obstructive sleep apnoea, chronic kidney disease or anorexia nervosa.7 In addition, certain medications, in particular glucocorticoids or opioids, reduce gonadotropins and testosterone levels.
Hyperprolactinaemia can lead to secondary hypogonadism through suppression of the pulsatile release of gonadotropin-releasing hormone (GnRH) from the hypothalamus. Hence, measurement of prolactin levels is essential and, if elevated, its cause should be investigated. If there is pathological hyperprolactinaemia, for example due to a microprolactinoma, the hypogonadism will respond to, and should be treated by, normalising the prolactin level rather than by testosterone therapy.
In the work-up for secondary hypogonadism, it is very important not to miss pituitary or hypothalamic pathology, which can damage gonadotroph production, resulting in hypogonadism. A careful clinical assessment for a pituitary mass effect (headache, visual field defect, eye movement disturbance), as well as for features of hypopituitarism or of a hormone producing pituitary tumour (eg. Cushing’s syndrome) must be undertaken. Haemochromatosis can be ruled out by measuring iron levels and determining the fasting transferrin saturation. Biochemical assessment of pituitary (dys-)function may be necessary, and pituitary imaging may need to be considered. Given that functional hypogonadism due to comorbidities presents with secondary hypogonadism, the yield of organic pathology is low in older obese men with comorbidities and only modest reductions in testosterone levels.
The practitioner needs to be aware that men with prior or current anabolic steroid use may present with low testosterone and LH levels and use may be denied even with specific questioning. Clues to androgen abuse are muscular build, a low HDL level or a higher than expected haematocrit; organic androgen deficiency is often associated with mild anaemia due to the erythropoietic actions of testosterone. The diagnostic approach to hypogonadism is summarised in Figure 1.
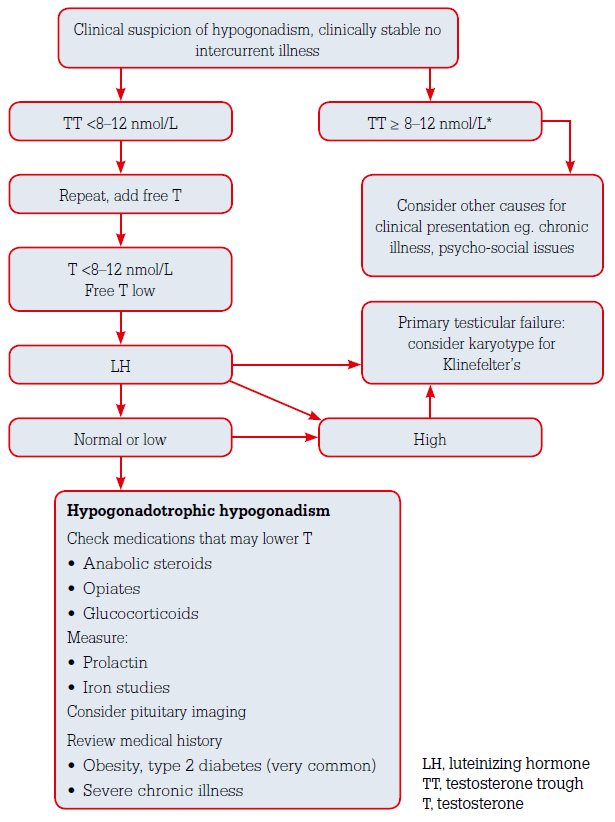
Figure 1. Work-up of androgen deficiency
Testosterone treatment
It is very important to remember that an underlying aetiology should always be sought before testosterone therapy is considered. This is not only because hypogonadism can be due to underlying pathologies such as a pituitary tumour or haemochromatosis, important diagnoses not to be missed, but also because gonadotropin treatment of secondary hypogonadism may restore fertility. Importantly, if testosterone therapy is commenced before the diagnosis is confirmed, the diagnostic work-up can be obscured and fertility compromised.
Testosterone replacement is recommended for symptomatic classical androgen deficiency syndromes after excluding contraindications in the initial work up (Table 2). Men with organic hypogonadism respond very well to testosterone replacement therapy and show a marked improvement in sexual function, sense of well being and energy levels, and maintenance of secondary sexual characteristics. However, testosterone therapy does not improve fertility; in fact, because of gonadotropin suppression, testosterone therapy may supress spermatogenesis. Indeed, testosterone therapy is being evaluated in trials of male contraception.
Table 2. Contraindications to testosterone therapy1
Breast or prostate cancer
A palpable prostate nodule or induration without further urological evaluation
PSA >4 ng/mL (or >3 ng/mL in men at high risk of prostate cancer) without further urological evaluation
Severe lower urinary tract symptoms (International prostate symptom score >19)
Haematocrit >50%
Untreated severe obstructive sleep apnoea
Uncontrolled or poorly controlled heart failure
When fertility is desired |
By contrast, in men with LOH, the distinction between replacement and pharmacological testosterone therapy becomes more difficult to define. Moreover, there is overlap between classical androgen deficiency and LOH. Certain men who have functional hypogonadism have profound androgen deficiency similar to men with organic hypogonadism due to intrinsic HPT axis pathology. The risk–benefit ratio of testosterone therapy in men with LOH is not well established because there are no large long-term randomised controlled clinical trials (RCT) that provide information about benefits for patients (eg. fracture reduction, improved functional morbidity or mortality) or therapy-associated risks. In men with functional hypogonadism resulting from chronic illness, especially obesity, the focus of therapy should be on lifestyle measures (especially weight loss) and optimisation of comorbidities. Although difficult to achieve and sustain, moderate weight loss (<10%) can increase testosterone levels by 2–3 nmol/L. Substantially larger increases (up to >10 nmol/) may be achieved in severely obese men who have successful bariatric surgery.6,7
Testosterone treatment in men with only modest reductions in circulating testosterone levels should be considered only if such measures fail, and is only recommended in the presence of symptoms. Symptoms of hypogonadism are non-specific and may be consequent to underlying comorbidities; men with symptomatic LOH are less likely to respond to testosterone therapy, compared with younger men with organic hypogonadism in whom such comorbidities are absent.7
There are no restrictions regarding testosterone replacement in men with organic hypogonadism due to established pathology of the HPT axis. By contrast, in men without classical androgen deficiency, testosterone therapy is subsidised by the Pharmaceutical Benefit Scheme (PBS) only if the testosterone level is <8 nmol/L or 8–15 nmol/L with high LH levels, defined as >1.5 times the upper limit of the eugonadal reference range for young men. However, the primary intent of this PBS cut-off of 8 nmol/L was to restrict unproven overuse of testosterone therapy, and even in men with repeated total testosterone levels of <8 nmol/L, treatment should be individualised.
It may be reasonable to consider a trial of testosterone therapy in selected men with LOH, in the absence of contraindications. As symptoms of androgen deficiency should improve within 1–3 months, a therapy trial of 3–6 months is usually of sufficient duration. It is important to inform the patient that this approach is not backed by high-level evidence of efficacy and safety. In addition, the patient should be informed at the outset that testosterone therapy will be stopped should there be no benefit, according to defined treatment goals agreed by the patient and practitioner. In such men, the therapeutic target should be to raise serum testosterone levels to the low-to-mid-normal range of healthy young men. The risk of iatrogenic suppression of the HPT axis with a short course of testosterone therapy is low, but longer acting testosterone preparations should be avoided initially. In men suitable for long-term testosterone therapy, different options are available (Table 3) and choice depends on patient and physician preference.
Androgen deficiency and erectile dysfunction are two overlapping conditions with distinct pathophysiology. A randomised, placebo-controlled trial studied the effect of testosterone therapy added to sildenafil in erectile dysfunction. The results showed that sildenafil plus testosterone was not superior to sildenafil plus placebo, although pre-treatment testosterone levels in such men were not unequivocally low.9
Table 3. Testosterone therapy: formulations1
| Preparation/ administration | Advantages | Disadvantages | Testosterone monitoring |
|---|
| Testosterone gel 1%, daily |
Can be self-administered
Short half-life in case of side effects |
Chance for inadvertent transfer to close contacts (spouse, children, nurses)
Imprecise dose adjustment
Marked variation in blood levels
Skin irritation |
Morning, before application, after use for 7 days |
| Testosterone patch, daily |
Can be self-administered
Short half-life in case of side effects |
Skin irritation
Limited scope for dose variation |
Morning after evening application |
| Testosterone transdermal solution, daily |
Can be self-administered
Short half-life in case of side-effects
Low chance of inadvertent transfer to others |
Pump applicator required
Must be administered in the axilla
Skin irritation
May not wash, shower or swim within 2 hours of application |
Monitor after 2 weeks, trough level taken 2–8 hours after application |
| Testosterone undecanoate 40 mg capsule, 2–3 times per day |
Can be self-administered
Short half-life in case of side effects |
Frequent dosing required (2–3 times daily)
Marked variation in blood levels
Gastrointestinal intolerance |
Monitor after 2 weeks |
| Testosterone undecanoate 1 g. IM injection 3-monthly |
Convenience
Compliance
Stable testosterone (T) levels |
Injection site pain
Contraindicated in men with coagulopathies
Cannot be self-administered |
Morning, before fourth injection. Aim for trough level 10–15 nmol/L. Allow a further 2–3 injections after dose adjustment before rechecking |
Testosterone implant 100 mg
6 monthly |
Convenience
Compliance
Stable T levels |
Invasive procedure required
Extrusion, infection, bleeding |
6-monthly after a 600–800 mg dose |
Testosterone treatment: potential risks and monitoring for adverse events
Young men with organic hypogonadism generally have a favourable risk–benefit ratio with testosterone therapy, provided they are monitored for side effects (Table 4) and men with contraindications (Table 2) have been excluded. By contrast, there may be increased risks in older, obese men because of comorbidities, such as prostate disease and undiagnosed obstructive sleep apnoea.1 RCTs in older men receiving testosterone therapy and meta-analyses to date have been underpowered to provide definitive outcome data regarding cardiovascular and prostate events. The main areas of concern relate to prostate and cardiovascular events. While there is no evidence that testosterone causes prostate cancer, the main practical issue is that because of prostate monitoring during testosterone therapy, there may be an increased risk of over-diagnosing pre-existing, clinically insignificant prostate cancer. One recent RCT has shown an increase in cardiovascular events with testosterone treatment in relatively frail older men,10 but numbers were small and may have been due to chance. Another RCT in a similar population has not confirmed this finding.11 Hence, monitoring patients according to a standardised monitoring plan is important.1
Table 4. Adverse effects of testosterone therapy1
| More common |
|---|
Erythrocytosis
Acne and oily skin
Detection of subclinical prostate cancer
Growth of metastatic prostate cancer
Reduced sperm production and fertility |
| Uncommon |
|---|
Gynaecomastia
Male pattern balding (familial)
Growth of breast cancer
Induction or worsening of obstructive sleep apnoea |
Key points
- Organic androgen deficiency due to intrinsic HPT axis pathology can be missed, and there is indeed evidence that classical androgen deficiency is underdiagnosed. Such men usually benefit markedly from testosterone replacement (except fertility), with a generally low risk of adverse events.
- Testosterone therapy should not be started without a thorough work-up to delineate the underlying aetiology and identify associated pathologies.
- Older obese men with chronic comorbidities commonly present with non-specific symptoms and modestly low testosterone. In such men emphasis should be on weight loss and optimisation of comorbidities. The risk–benefit ratio of testosterone therapy in such men is less favourable than in men with organic androgen deficiency.
- Testosterone therapy should include:
- a discussion about the uncertainties regarding risks/benefits (especially in men without organic androgen deficiency)
- patient-centred goals
- implementation of a standardised monitoring program
- ongoing evaluation for benefit/ adverse events.
- Large, well-conducted clinical trials are needed to provide more evidence to guide clinicians and patients regarding the benefits, and risks, of testosterone therapy.
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.