Male patients were three times more likely than female patients, and patients aged ≥45 years were seven times more likely than younger patients to have cardiomyopathy managed (Figure 1). The most common other problems managed with cardiomyopathy were diabetes, atrial fibrillation and hypertension.
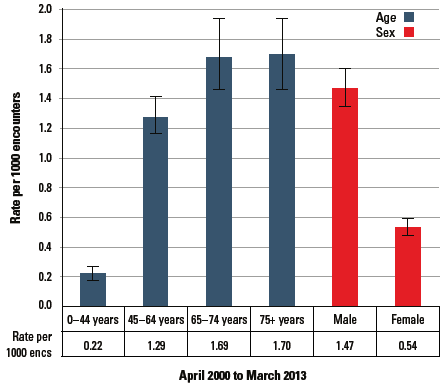
Figure 1. Cardiomyopathy: Rate per 1000 encounters (95%CI)
Management of cardiomyopathy
There were 115 medications recorded per 100 cardiomyopathy problems, which is a higher rate than average for all encounters in BEACH (69 per 100 problems). Frusemide and carvedilol were each recorded at a rate of 17 per 100 cardiomyopathy problems managed. Other treatments were recorded at a rate of 21 per 100 cardiomyopathy problems managed, lower than the average rate of 35 per 100 total problems. They comprised clinical treatments, almost all of which were counselling or advice and procedures, the most common of which were INR tests at point of care. Referrals were provided at a rate of 11 per 100 cardiomyopathy problems and most were to cardiologists. Pathology tests were ordered often (40 per 100 cardiomyopathy problems), but imaging orders were infrequent (Table 1). Cardiomyopathy is one of the less commonly managed problems in general practice and the management rate has remained steady over the past 13 years.
Table 1. Treatments for cardiomyopathy
| Treatment | Number of treatments | Rate per 100 cardiomyopathy problems | Proportion of each treatment type (%) |
|---|
|
Medications
Frusemide
Carvedilol
Warfarin
Perindopril
Ramipril
|
1351
202
194
116
87
82
|
115.0
17.2
16.5
9.9
7.4
7.0
|
100.0
15.0
14.4
8.6
6.4
6.1
|
|
Other treatments
Advice medication
Counselling
INR tests
|
251
41
40
22
|
21.4
3.5
3.4
1.9
|
100.0
16.3
15.9
8.8
|
|
Referrals
Cardiologist
|
131
112
|
11.1
9.5
|
100.0
85.5
|
|
Pathology tests
EUC
Full blood count
Coagulation
|
471
89
72
71
|
40.1
7.6
6.1
6.0
|
100.0
18.9
15.3
15.1
|
|
Imaging
Echocardiography
|
45
29
|
3.8
2.5
|
100.0
64.4
|
Acknowledgements
The authors thank the GP participants in the BEACH program, and all members of the BEACH team. Funding contributors to BEACH from April 2000 to March 2013: Australian Government Department of Health and Ageing; AstraZeneca Pty Ltd (Australia); CSL Biotherapies Pty Ltd; Merck, Sharp and Dohme (Australia) Pty Ltd; National Prescribing Service; Novartis Pharmaceuticals Australia Pty Ltd; Pfizer Australia Pty Ltd; Abbott Australasia Pty Ltd; Janssen-Cilag Pty Ltd; Sanofi-Aventis Australia Pty Ltd; GlaxoSmithKline Australia Pty Ltd; Bayer Australia Ltd; Wyeth Australia Pty Ltd; and Roche Products Pty Ltd. BEACH is approved by the Human Research Ethics Committee of the University of Sydney. Authors
Competing interests: None.
Provenance and peer review: Commissioned; not peer reviewed.