Ximelagatran, the first of the alternative agents to be developled, is a direct thrombin inhibitor. It showed clinical efficacy in non-valvular atrial fibrillation and venous thromboembolic disease in studies conducted in 2000–2005. It was approved for both indications in a range of countries throughout Europe but it was associated with an unacceptable incidence of liver toxicity and was withdrawn from the market in 2006. Ximelagatran was never approved for use in Australia.
A number of new oral anticoagulants (NOACs) with properties that overcome the practical limitations of warfarin have recently become available. These agents have a more stable pharmacokinetic profile, have no significant food–drug interactions and fewer drug–drug interactions, and can be administered in a standard dose without the need for routine monitoring.
The NOACs have been evaluated for use in venous thromboembolic disease, non-valvular atrial fibrillation and several other cardiac indications. Three NOACs now have Therapeutic Goods Administration (TGA) approval for use in Australia and are listed on the Pharmaceutical Benefits Scheme (PBS) for subsidy. The purpose of this article is to provide a simple overview of the different agents and some rational guidance on their integration into our clinical practice.
Pharmacology of the NOACs
The NOACs fall into two broad categories: direct thrombin inhibitors and the factor Xa inhibitors (Figure 1). The direct thrombin inhibitors inactivate soluble and fibrin-bound thrombin and limit thrombogenesis and thrombus growth.1 Dabigatran, the second orally active direct thrombin inhibitor to be marketed after ximelagatran, is not associated with hepatotoxicity and has been approved for stroke prevention in atrial fibrillation and prevention of venous thromboembolism in at-risk populations. Factor Xa inhibitors directly inhibit the enzyme responsible for thrombin formation.2 Those available include apixaban and rivaroxaban. The properties of the different NOACs are shown in Table 1.
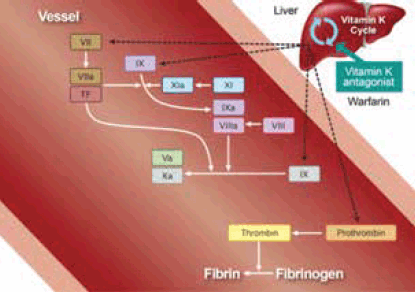
Figure 1. Sites of action of warfarin
Reproduced with permission from Elsevier Ltd from Uchiyama S, Ibayashi S, Matsumoto M, et al. J Stroke Cerebrovasc Dis 2012;21:165–73
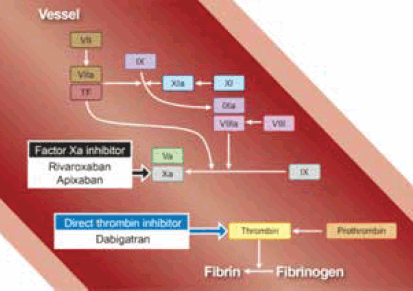
Figure 2. Sites of action of the NOACs
Reproduced with permission from Elsevier Ltd from Uchiyama S, Ibayashi S, Matsumoto M, et al. J Stroke Cerebrovasc Dis 2012;21:165–73
Table 1. Properties of the NOACs
| Property | Apixaban | Dabigatran (as etexilate) | Rivaroxaban |
|---|
| Target |
Xa |
IIa (thrombin) |
Xa |
| Bioavailability (%) |
50 |
6.5 |
66 |
| Cmax (hours) |
3–4 |
0.5–2.0 |
2–4 |
| t1/2 (hours) |
12 |
12–14 |
11–13 |
| Dosing |
bid |
bid |
od |
| Metabolism |
P-gp
Cyp3A4 |
P-gp |
P-gp
Cyp3A4 |
| Renal excretion |
27% |
85% |
36% |
| Cmax, maximum plasma concentration; t½, plasma half life; P-gp, permeability glycoprotein; Cyp3A4, cytochrome P450 3A4 enzyme |
Clinical indications
Venous thromboembolic disease
Prophylaxis
Although dabigatran, rivaroxaban and apixaban are available in Australia for primary venous thromboembolism (VTE) prophylaxis (Table 2), approval is limited to the context of elective hip and knee replacement surgery, where they have been extensively studied. Dabigatran (150 mg and 220 mg once daily) was as effective as enoxaparin 40 mg daily at preventing any VTE and mortality of any cause, with no significant difference in major bleeding rates.3 Rivaroxaban 10 mg daily and apixaban 2.5 mg twice daily were each superior to enoxaparin 40 mg daily with no difference in major bleeding rates.4–6 Currently, there are no major studies evaluating the use of NOACs in hip fracture surgery, minor orthopaedic procedures and non-orthopaedic surgery. Consequently, they are not approved for these uses.
NOACs do not have TGA approval for prophylaxis in acutely ill medical inpatients at risk of VTE, although rivaroxaban (MAGELLAN trial) and apixaban (ADOPT trial) have been studied.7,8 Rivaroxaban 10 mg given daily for 35 days was superior to enoxaparin 40 mg for 6–14 days, and apixaban 2.5mg twice daily for 30 days was non-inferior to enoxaparin. Both NOACs showed increased bleeding risk when used for these extended periods of thromboprophylaxis.7,8
Table 2. Summary of current TGA approved indications for warfarin and individual NOACs
| Clinical indication | Warfarin | Apixaban | Dabigatran | Rivaroxaban |
|---|
| VTE prophylaxis following elective hip or knee surgery |
Yes |
Yes |
Yes |
Yes |
| VTE prophylaxis in acutely ill medical at-risk inpatients |
No |
No |
No |
No |
| VTE prophylaxis for surgery following hip fracture, minor orthopaedic or non-orthopaedic procedures |
No |
No |
No |
No |
| VTE treatment |
Yes |
No |
No |
Yes** |
| Thromboprophylaxis for non-valvular atrial fibrillation |
Yes |
Yes |
Yes |
Yes |
| Thromboprophylaxis for patients with significant valve disease* and atrial fibrillation |
Yes |
No |
No |
No |
| Thromboprophylaxis for patients with mechanical prosthetic cardiac valve replacement |
Yes |
No |
No |
No |
VTE, venous thromboembolism
* Mitral stenosis, bioprosthetic heart valve or mitral valve repair24
** Excluding patients with active cancer or antiphospholipid syndrome |
Treatment and secondary prevention
Only rivaroxaban is currently approved and subsidised in Australia for treatment of DVT and PE. Its use offers a convenient, single-drug approach to VTE treatment. Its efficacy is comparable to that of warfarin for prevention of recurrent VTE but it has a lower bleeding risk (Table 3). High-risk patients, such as those with antiphospholipid syndrome and recurrent thrombotic events, were excluded from clinical trials and warfarin should remain the standard of care for these patients until NOACs have been evaluated.9,10 Apixaban and dabigatran have also been studied in VTE treatment and may eventually become available for this indication (Table 3). All NOACs are small molecules that cross the placenta and are contraindicated in pregnant and breastfeeding patients.
Dabigatran, rivaroxaban and apixaban were evaluated for extended (6–12 months) treatment of VTE and, as expected, showed reduced risk of recurrence, compared with placebo. However, only dabigatran was compared with warfarin, the current standard of care, and had comparable results for VTE recurrence and bleeding rates.10–12 Although rivaroxaban is available and subsidised by the PBS for extended secondary VTE prevention, there are no data to indicate it is equivalent to warfarin for long-term prevention of recurrent VTE in high-risk patients. The risk–benefit ratio of continued anticoagulant therapy should be re-assessed at least annually.
Table 3. DVT treatment and extension trials
| Rivaroxaban | Apixaban | Dabigatran |
|---|
| Trial name |
EINSTEIN DVT/extension10
EINSTEIN PE9 |
AMPLIFY20 |
RECOVER I21
RECOVER II22 |
| Dose |
15 mg bd x 3 weeks then 20 mg od |
5 mg bd |
150 mg bd |
| Design |
Open-label |
Blinded |
Blinded |
| Initial heparin |
No |
No |
Yes |
| VTE recurrence (relative risk) |
0.89 (0.66–1.19) |
0.84 (0.60–1.18) |
1.10 (0.65–1.84)
1.08 (0.64–1.80) |
| Major bleeding (relative risk) |
0.54 (0.37–0.79) |
0.31 (0.17–0.55) |
0.82 (0.45–1.48)
0.69 (0.36–1.32) |
| TTR for warfarin |
62% |
61% |
60% |
| TTR, time in therapeutic range |
Atrial fibrillation
Each of the three NOACs available in Australia has been evaluated in patients with non-valvular atrial fibrillation and at least one additional risk factor for stroke in single, large multicentre trials (Table 4).13–15 A direct comparison of the results of the three major trials is impeded by differences in trial design, patient populations, definitions of endpoints and availability of published data for some endpoints. Nevertheless, some consistent themes have emerged and can be summarised as follows:
- All NOACs are ‘non-inferior’ to warfarin in the primary efficacy endpoint of stroke and systemic embolism. Apixaban and dabigatran 150 mg are superior to warfarin, although the numbers needed to treat to show this benefit are large (number needed to treat with a NOAC rather than warfarin to prevent one stroke: 312 for apixaban; 175 for dabigatran 150 mg).
- All NOACs are ‘non-inferior’ to warfarin for the primary safety endpoint of major bleeding. Apixaban and dabigatran 110 mg are superior to warfarin. Intracranial haemorrhage, an infrequent but serious complication of warfarin, is significantly less frequent with all NOACs. Again, the numbers needed to treat to reduce this important outcome are large (196 for dabigatran 110 mg; 500 for rivaroxaban).
Table 4. Phase III atrial fibrillation trials
| Apixaban | Dabigatran | Rivaroxaban |
|---|
| Trial Name |
ARISTOTLE14 |
RE-LY13 |
ROCKET-AF23 |
| Dose (mg) |
5 (2.5**) |
150, 110 |
20 (15*) |
| Freq |
bid |
bid |
qd |
| N |
18 206 |
18 113 |
14 266 |
| Design |
2x blind
Non-inferiority |
PROBE
Non-inferiority |
2x blind
Non-inferiority |
| AF criteria |
AF or AFl x2
<12 months |
AF x1
<6 months |
AF x2
(>1 in <30 days) |
| % VKA naive |
43 |
50 |
38 |
*Dose adjusted in patients with reduced drug clearance.
** Dose adjusted in patients with two or more of: reduced drug clearance, low body weight, elderly
***Dose adjusted in patients with reduced drug clearance, low body weight, concomitant use of potent P-glycoprotein inhibitors. AF = atrial fibrillation; AFl = atrial flutter; x1 = previous episode; x2 = previous episodes; PROBE = prospective, randomised, open-label, blinded end-point evaluation; VKA = vitamin K antagonist |
Choosing between warfarin and a NOAC
Many patients find the limitations of warfarin burdensome and attend their GP requesting a change to a NOAC. For patients on warfarin whose INR levels are easily maintained within target levels, the clinical benefit of such a change is limited; the main reason for choosing a NOAC under this circumstance is patient preference. For patients in whom INR control is difficult, however, alternative strategies, such as home-monitoring of INR or change to a NOAC, should be considered.
All of the NOACs have some degree of renal excretion so for patients with severe renal impairment or labile renal function, warfarin should remain the anticoagulant of choice. The NOAC trials included patients with moderate chronic kidney disease and dose adjustment algorithms have been developed to optimise NOAC use in this population (Table 5).
Clinical experience with NOACs is limited, compared with warfarin, and the role of NOACs in the broader range of patients than those included in the clinical trials is uncertain. Rare adverse events may yet be encountered and reporting of any events associated with the use of NOACs to the TGA is important. Post-marketing surveillance will provide more information about this in the future.
Table 5. NOAC dose modification in the atrial fibrillation trials
| Drug | Renal excretion | Indications for dose reduction | Renal function contraindication |
|---|
| Apixaban |
27% |
If 2 or more of:
- age ≥80 years,
- body weight ≤60 kg, or
- serum Cr of ≥133 µmol/L
lower dose to 2.5 mg bd |
CrCl <25 mL/min |
| Dabigatran |
85% |
If CrCl 30–50 mL/min: lower dose to 110 mg bd |
CrCl <30 mL/min |
| Rivaroxaban |
36% |
If CrCl 30–49 mL/min: lower dose to 15 mg |
CrCl <30 mL/min |
| CrCI, creatinine clearance |
Choosing between NOACs
Factors to consider when deciding between the NOACs include the patient’s likelihood to comply with twice daily (dabigatran, apixaban) versus single, daily (rivaroxaban) treatment, and any concomitant chronic medications that may interfere with the metabolism of the drugs. For practical purposes, the most important interactions are with verapamil and amiodarone, which can increase the circulating concentrations of all three NOACs. This effect is minimised by ingestion of the anticoagulant drug at least 2 hours before ingestion of the other medications. Rivaroxaban and apixaban should not be co-administered with azone antifungals or HIV protease inhibitors.
In the atrial fibrillation studies, gastrointestinal bleeding was encountered more frequently with dabigatran and rivaroxaban than with warfarin; this was not the case for apixaban. If a patient has a predisposition to this condition (untreated ulcer symptoms, previous gastrointestinal bleeding with non-remediated cause), apixaban or warfarin may be more prudent options.
Other potential indications
Atrial fibrillation in patients with valvular heart disease
Patients with atrial fibrillation and haemodynamically significant valvular heart disease were excluded from the trials evaluating the NOACs. Warfarin remains the standard of care for these patients and, until further studies are performed, NOACs are not recommended for patients with atrial fibrillation and valvular heart disease.
Prosthetic valve thromboprophylaxis
Patients with prosthetic valves represent a particularly high-risk group for whom warfarin has been the mainstay of therapy. To date, only dabigatran has been evaluated in this population. However, the study was terminated prematurely because of an increased rate of thromboembolism and bleeding in patients receiving dabigatran.16 It is important that patients in this group not be treated with a NOAC.
Laboratory testing
Although NOACs do not require routine monitoring, laboratory testing is informative in the context of bleeding, urgent surgery or recurrent thromboembolism. Standard coagulation assays are variably affected by NOACs but cannot provide drug quantification and results are not equivalent to INR testing for warfarin.17–19 At present, assays for drug quantification (Table 6) are performed in specialised coagulation laboratories with advice from a haematologist.
Table 6. Effect of the NOACs on routinely performed coagulation assays
| Dabigatran | Rivaroxaban | Apixaban |
|---|
| Significant anticoagulant effect unlikely |
APTT and TT normal |
PT normal |
Normal PT DOES NOT exclude presence of therapeutic apixaban |
| Anticoagulant effect present |
TT prolongedAPTT prolonged |
PT prolonged |
PT prolonged or normal |
| Specific assays to quantify drug presence |
Dilute thrombin clotting time (Hemoclot assay) |
Modified Anti Xa assay specific for rivaroxaban |
Modified Anti Xa assay Specific for apixaban |
| APTT, activated partial thromboplastin time; TT, thrombin time |
Management of bleeding
The cause of bleeding should be evaluated and the presence of residual or excessive anticoagulant effect assessed. Minor bleeding may be managed with local measures and temporary drug cessation. Patients with clinically significant bleeding may be managed with charcoal, standard resuscitation measures and surgical, radiological or endoscopic intervention. Prohaemostatic agents may be used but have no proven efficacy. Dabigatran may be removed with dialysis but factor Xa inhibitors are too highly protein-bound. Vitamin K does not reduce the anticoagulant activity of NOACs and is of no benefit. Reversal agents have now been developed for NOACs but they are just entering clinical trial evaluation. Management of bleeding often requires expert haematological advice and GPs should ensure they have ready access to these services when commencing patients on a NOAC.
Conclusions
The more selective mechanisms of action of the NOACs and the fact that they do not require routine laboratory monitoring make them viable alternatives to warfarin for many, but not all, conditions requiring anticoagulant therapy. The NOACs are contraindicated in patients with end-stage renal failure and should be used carefully in patients with renal impairment. Given that experience with these agents is limited, prescribers need to be vigilant for adverse events and report these to the TGA. If required in cases of urgent surgery or bleeding, laboratory monitoring can be performed in specialised laboratories in most teaching hospitals. For currently approved indications, bleeding risk with NOACs is not increased when compared with warfarin. When clinically significant bleeding does occur, it should be managed in conjunction with specialist haematology advice. Currently, no antidotes are available; however, reversal agents for each drug have been developed and are undergoing evaluation in clinical trials.
Key points
- The main difference between the NOACs (apixaban, dabigatran and rivaroxaban) and warfarin is that routine laboratory monitoring of coagulation is not required for the NOACs.
- The NOACs are indicated for thromboprophylaxis in patients with non-valvular atrial fibrillation and should be considered in patients who are poorly controlled on warfarin or who express a strong preference for one of the newer drugs.
- All agents may be used for thromboprophylaxis following hip and knee replacement surgery and rivaroxaban can be used for treatment and secondary prevention following venous thromboembolic events.
- The NOACs are not indicated for other conditions, including anticoagulation following mechanical prosthetic valve replacement, hip fracture surgery, minor orthopaedic procedures and non-orthopaedic surgery, or prophylaxis in acutely ill medical inpatients at risk of VTE
- Standard coagulation assays respond variably to the different NOACS and laboratory testing, if required is best done in specialised haematology laboratories with advice from a haematologist.
- Given the limited experience with NOACs, there is the potential for as yet unrecognised adverse events and reporting of suspected events to the TGA is important with these new agents.
Competing interest: David Brieger received honoraria as an independent consultant on advisory boards regarding the NOACs for Boerhinger Ingelheim, Bayer and BMS/Pfizer.Jenny Curnow received speakers honoraria from Boehringer Ingelheim and Bayer, and participated in advisory boards for BI and Pfizer. She received support from BI, Bayer and Pfizer to attend conferences.
Provenance and peer review: Commissioned, externally peer reviewed.