General points
The reader is referred to the November 2012 AFP article ‘MRI of the knee’ for a general discussion of MRI.2
The examination is relatively long and takes 15–30 minutes to complete. The patient needs to remove any metal and lie flat within the bore or ‘tunnel’ of the magnet. The head and neck are enclosed within a rigid radiofrequency coil; this coil does not touch the patient but is close to the face. The patient is still able to see out and can speak to the MRI technologist at all times. The patient must wear headphones to suppress noise and these are used to play the patient’s favourite music or radio station streamed via the internet. An injection of contrast through an intravenous cannula may be required.
Contraindications and precautions
Implants containing metal may move in the MRI system and the function of implants can be permanently altered. Table 1 gives a guide to common contraindications and precautions to MRI scanning.3 Interference can occur outside the scanner as the magnetic field extends beyond the scan room. Patients are screened for the presence of these devices before entering the MRI suite and some examinations may need to be cancelled or deferred; it is very useful for the MRI service to be aware of these devices before the patient’s appointment, to avoid delays to the patient.
Table 1. Contraindications to MRI3
| Absolute contraindications | Relative contraindications* |
|---|
- Cardiac pacemaker or defibrillator
- Metallic foreign body in eye
- Deep brain stimulator
- Aneurysm clips (if ferrous)
- Gunshot close to vital structures
- Cochlear implant
- Drug infusion devices
- Swan-Ganz catheters
- Some dental implants
|
- Stents, including abdominal aortic aneurysm, recently inserted
- Stapes implant
- Drug-infusion pump
- Neuro- or bone-growth stimulator
- Ocular prosthesis
- Penile prosthesis
- Any implant or mechanical device
|
| *Need to be assessed as safe before patient enters MRI suite |
GPs should contact their MRI service for advice before denying a patient an MRI scan, for example, because of a pacemaker. Some devices may be safe to scan after appropriate investigation even when an ‘absolute contraindication’ is present. Death has occurred when patients with cardiac pacemakers were placed in MRI systems. Some pacemakers are now MRI compatible but need to be switched off by a cardiac technologist before scanning, requiring coordination with the patient’s cardiologist. Many devices have not been fully tested in higher-field strength systems (eg. 3.0 tesla (T) scanners) and may need to be scanned at lower-field strength (1.5T).
Patients of very high body mass index may not fit within the bore of a conventional magnet. Some systems offer a wider bore that is also better tolerated by claustrophobic patients. Patients with severe claustrophobia and children under the age of 6 years will generally need sedation under the supervision of an anaesthetist. Pregnancy is no longer a relative contraindication but pregnant patients should not receive gadolinium.
MRI contrast and nephrogenic systemic fibrosis (NSF)
The most common contrast medium used in MRI is gadolinium, a paramagnetic metal ion that shortens T1 relaxation time, resulting in increased T1 signal. Gadolinium is chelated to allow safe excretion, as free gadolinium is highly toxic to tissues. In severe renal disease, biological half-life is increased and the gadolinium chelate can dissociate, resulting in binding of free gadolinium in tissues by displacing endogenous metals, including iron and calcium. Phagocytosis by macrophages is believed to induce cytokine release, which results in fibrosis and the irreversible and debilitating clinical syndrome of contractures and pain.4 Patients on haemodialysis should not receive gadolinium-based contrast. Individuals with stage 3 kidney disease (eGFR 30–60 ml/min/1.73 m2) have a very low risk of NSF. The risk of NSF per injection rises from 0.1% in stage 4 disease (15–30 ml/min/1.73 m2) to 1% in stage 5 (<15 ml/min/1.73 m2),5 and most MRI centres will avoid gadolinium in these groups.
Indications
MRI is the gold standard for investigation of most diseases of the central nervous system (CNS)and as such the indications are wide, including stroke, temporal lobe epilepsy, tumour, infection, inflammation, multiple sclerosis, dementia, metabolic disorders, post-trauma, congenital malformations, nerve palsies, internal auditory canal masses, vascular diseases and pituitary pathology.3 In acute presentations, such as trauma and stroke, computerised tomography is still the preferred primary investigation as it is faster and allows decisions regarding interventions such as surgery or thrombolysis to be expedited.
Reading MRI
GPs and other medical specialists are increasingly interested and adept at reading medical imaging studies. MRI, with its multiplicity of imaging sequences for different indications, takes more time to become used to but can be conquered. The sequences most commonly used in MRI of the brain are T1 (used broadly for anatomical definition and tissue characterisation), T2 (generally showing oedema and pathology as high or ‘white’ signal), fluid-attenuated inversion recovery (FLAIR; suppressing cerebrospinal fluid signal and making ‘white’ pathology more conspicuous), susceptibility or gradient echo imaging (sensitive to degraded blood products) and diffusion-weighted imaging (showing altered water motion and most commonly used to diagnose acute stroke). Post-contrast imaging is T1-weighted, usually with fat suppression, to highlight integrity of the blood–brain barrier.
Multiple sclerosis
The characteristic features of multiple sclerosis (MS) on MRI are T2 hyperintense foci in the cerebral white matter, which represent areas of perivenular demyelination (Figure 1). ‘Dawson’s fingers’ are plaques extending perpendicularly from the surface of the lateral ventricles like open fingers. MRI can be used to confirm the clinical suspicion of MS when dissemination in time and space is shown according to the revised McDonald criteria6. Dissemination in time is demonstrated when acute hyperenhancing and chronic non-enhancing plaques are present (allowing a diagnosis to be confirmed on a single scan), or when a follow-up scan shows at least one new plaque. Dissemination in space is present when there is at least one plaque in two or more characteristic locations: juxtacortical, periventricular, infratentorial or spinal cord.6
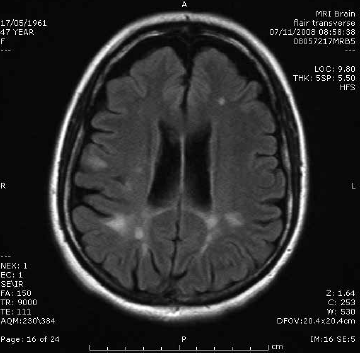
Figure 1. Multiple sclerosis. Sagittal T2 FLAIR image showing characteristic periventricular demyelination plaque radiating perpendicularly from the ventricular surface
White matter T2 hyperintensities
Multiple small T2 ‘bright spots’ (Figure 2) are commonly identified incidentally on MRI and can be a diagnostic dilemma. These foci are more frequently identified with increasing age but are also associated with hypertension, increasing in severity where hypertension is uncontrolled.7 A general rule of thumb is that patients will have one bright spot per decade of life. Patients should be assessed for cardiovascular risk factors and blood pressure interventions may be required, particularly if there is also evidence of large vessel or cortical stroke. In younger patients without cerebrovascular disease risk factors, the aetiology of these lesions may be unclear. Clinical correlation with features of demyelinating disease and follow up is usually recommended if there are more foci than normal for age. Neurological referral may also be helpful in unclear cases.
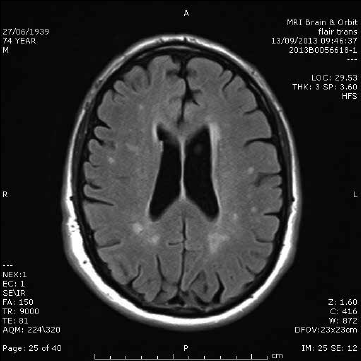
Figure 2. White matter disease. Axial T2-weighted image showing multiple small T2 hyperintensities with random distribution
Stroke
MRI can be used in diagnosis of acute (Figure 3) and chronic stroke. In diffusion-weighted imaging, image contrast is due to differing rates of water motion in tissues. In acute ischaemia there is disruption of the sodium–potassium pump, resulting in net movement of water from the extracellular to intracellular space. Diffusion-weighted imaging can identify hyperacute stroke within 30 minutes of onset of symptoms as an area of bright signal. It is also useful in differentiating cerebral infection from tumour and demyelination.8
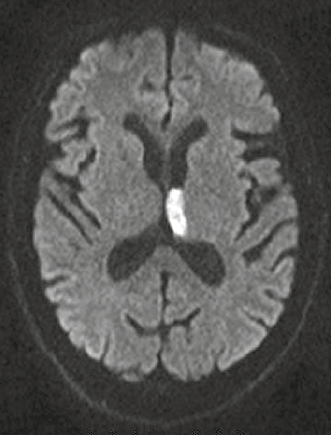
Figure 3. Diffusion-weighted imaging showing acute (white signal) infarct involving the left thalamus
Tumour
MRI is used in the investigation of a suspected tumour, characterisation of tumour type and in follow up of known lesions (Figure 4). Imaging is an integral tool in determining final tumour grade. The location, morphology, composition and enhancement are used to narrow the differential diagnosis and guide management. An intra-axial mass arises in the cerebral substance, in grey or white matter; extra-axial locations include the cerebrospinal fluid spaces, leptomeninges and cranial nerves. The position of the lesion above or below the tentorium, and patient age help to further refine the differential. Some lesions characteristically contain calcification or haemorrhage. Enhancement indicates breakdown of the astrocyte processes and vascular tight-junctions, which form the blood–brain barrier, and is associated with more aggressive tumours.
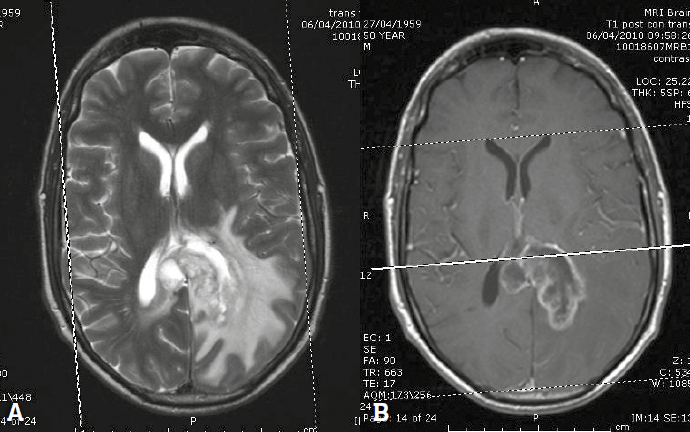
Figure 4. Glioblastoma multiforme. A. T2 weighted image showing a heterogeneous mass in the left parieto-occipital lobe. B. Post-contrast T1 weighted image showing ring enhancement
Symptoms of large tumours are often due to mass effect on surrounding structures or displacement of uninvolved structures. Oedema can be either cytotoxic (due to intracellular shift of water) or vasogenic (due to increased vascular permeability with failure of the blood–brain barrier). In most cases, both types of oedema are present. Vasogenic oedema is characterised by spread along white matter tracts, sparing grey matter. In cytotoxic oedema the blood-brain barrier is intact and oedema is caused by failure of the sodium–potassium pump; this type of oedema affects grey and white matter.
The most common intracerebral tumour in adults is metastatic disease. In many cases the presence of multiple lesions will establish the diagnosis of metastatic disease where there is a known history, but biopsy is still required in many patients. Figure 4 shows a typical high-grade primary glioblastoma multiforme on T2: a poorly defined heterogeneous mass with abundant surrounding vasogenic oedema of slightly less intense signal; following contrast, the lesion shows irregular ring enhancement. Other ring enhancing lesions include metastatic disease or cerebral abscess.
Aneurysm
Magnetic resonance angiography (MRA) is a sensitive method for detecting aneurysms in the circle of Willis and does not require gadolinium contrast. Instead it uses a property of red blood cell motion to form the image (Figure 5). MRA sensitivity is very high (99%) at higher-field strength (3T)9 but can be reduced at lower-field strength (1.5T) when an aneurysm is thrombosed or if the aneurysm is small (<3 mm).10
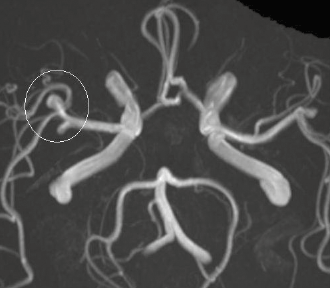
Figure 5. Cerebral aneurysm. MRA image of the circle of Willis showing an aneurysm in the right middle cerebral artery (white circle)
Vestibular schwannoma
MRI shows exquisite detail of skull base structures and is the preferred first-line tool in screening of patients with asymmetrical hearing loss for the presence of ‘acoustic neuroma’ (Figure 6). Schwannomas arise from the vestibular portion of the eighth cranial nerve and when small are intracanalicular (or completely contained within the internal auditory canal). A large schwannoma (Figure 6) will fill the cerebello-pontine angle and will need to be distinguished from meningioma. MRI can also identify other causes of hearing loss, including semicircular canal dehiscence, middle ear pathology and congenital abnormalities.
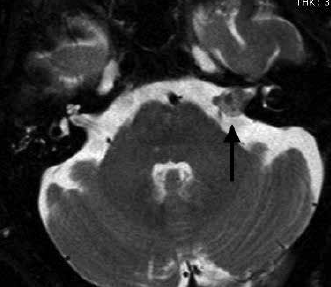
Figure 6. Vestibular schwannoma. T2 weighted axial image showing enlargement of the eighth cranial nerve in the internal auditory canal (arrow).
Conclusion
MRI of the brain is the gold standard for investigation of most disorders of the CNS. Many GPs will find a working understanding of common MRI imaging sequences and findings useful in explaining diagnoses to their patients. Some patients, including most patients with cardiac pacemakers, are still excluded from MRI imaging. Assessment of renal function in patients at risk of renal disease is essential prior to most MRI brain imaging to alert the MRI centre of risk of nephrogenic systemic fibrosis.
Competing interests: None.
Provenance and peer review: Commissioned; externally peer reviewed.