Osteoarthritis as a public health problem
Osteoarthritis is the most common form of arthritis and the incidence is increasing markedly due to an ageing population. It is a whole-joint problem that leads to cartilage loss and, eventually, joint failure. In Australia, it is one of the most frequent causes of pain, loss of function and disability in adults. It is pain that drives the patient to seek help, interferes with quality of life and accounts for up to 30% of the variance in quality-of-life scores in people aged 50–80 years living in Hobart.1 The aim of this article is to review the literature on factors associated with pain and discuss the implications of these findings for therapy, with a focus on the knee.
Radiographs and knee pain
There have been many studies of knee pain.2 A systematic review of the literature showed that 15–76% of patients with knee pain were found to have radiographic osteoarthritis, and 15–81% of those with radiographic knee osteoarthritis had pain.2 Overall, there is a modest but significant correlation between the degree of radiographic change and knee pain, which is most consistent for osteophytes.2 However, a study from our group reported a significant association between osteophytes and pain but this disappeared after adjustment for muscle strength, BMI and a number of factors assessed on MRI scanning,3 suggesting that osteophytes may be a reflection of the disease process but not a key player.
MRI features and knee pain
Bone marrow lesions
There is strong and increasing evidence from studies in humans that bone has an important role in the pathogenesis of osteoarthritis.4 In particular, bone marrow lesions (BMLs), have been recognised as a key feature of knee osteoarthritis.5–7 A number of studies have linked BMLs with knee pain.3,5–7 There are now two papers that show a significant correlation between change in BMLs and change in pain, both in unselected community living6 and osteoarthritis populations,7 suggesting a potential target for therapy. Indeed, a report of a proof-of-principle trial done in those with only BMLs demonstrated that zoledronic acid (a potent bone-acting bisphosphonate) could decrease both pain and BML size over 6 months.8 Another trial of chondroitin sulphate in patients who had knee osteoarthritis showed a decrease in BML size over 12 months but no change in pain.9
Cartilage defects
Cartilage defects are very common, being present in high proportions in many studies.4 Cartilage is aneural and therefore defects in cartilage should not be associated with pain. Despite this, there is consistent evidence that they are associated with pain,3,10–14 and that this is largely independent of other structural factors. Cartilage defects correlate strongly with BMLs, suggesting that BMLs and cartilage defects are closely related but both have independent associations with pain. This may be mediated by substance P nociceptive fibres15 or superinduction of cyclooxygenase 2 and prostaglandins.16 Weight loss may improve defects.17
Meniscal pathology
The association between meniscal pathology and pain remains controversial and may reflect site-specific associations. Any meniscal damage was associated with knee pain but not after adjustment for radiographic osteoarthritis.18 In a study from our institution, meniscal tear at the lateral posterior and anterior horns, but not other sites, was significantly associated with questionnaire-assessed pain, stiffness and function scores.19 In another study, macerated tears were associated with pain.14 Again, weight loss may improve cartilage loss in those with meniscal tear.20
Inflammation
The evidence links local inflammation (measured as synovitis and/or effusions) with pain.21 Further, in two studies, changes in synovitis (but not effusion severity) were associated with fluctuations in knee pain in patients with knee osteoarthritis,22,23 suggesting that synovitis may be the key factor and effusion a consequence of synovitis. C‑reactive protein (CRP) levels in serum are also predictive of knee pain development over 5 years24 (even though they are within the normal range) and this association is independent of all measured knee structural abnormalities in that study, suggesting systemic inflammation is also important. In terms of therapy, this opens up a number of options. Corticosteroid injections are effective for pain in knee osteoarthritis but seem to be most effective for effusions.25 Thus, it would seem logical to preferentially give corticosteroid injections to those with effusions. Non-steroidal anti-inflammatory drugs (NSAIDs) help to manage pain in osteoarthritis but there is limited data on who may benefit most from the use of these agents.26
Other knee structures
There is generally consistent evidence that knee bone size, subchondral bone density, meniscal extrusion and cartilage volume are not associated with pain3 (also Jones G et al, unpublished data).
Obesity
Obesity is a very strong, independent correlate of knee pain and is consistently associated with knee pain in many studies.3,11,27 There is Level 1 evidence that weight loss improves knee symptoms,26 although interestingly, there is a stronger correlation between weight gain and worsening pain than between weight loss and improvements in pain, suggesting limited reversibility.28 Nevertheless, in the overweight patient with limited structural pathology, weight loss should be the main objective.
Weak muscles
Similarly to obesity, muscle weakness, especially in the quadriceps, is independently associated with pain in cross-sectional3 and longitudinal studies.29 There is Level 1 evidence that strengthening and aerobic exercises help to manage pain in knee and hip osteoarthritis.26 Whether these therapies work better in those with weaker muscles is unknown.
Central factors and genetics
It is clear that pain in osteoarthritis is also modulated by a number of central factors, such as depression, catastrophisation (the tendency to view or present a situation as considerably worse than it actually is), self-efficacy and a positive attitude.30–32 Recently, there have been studies implicating specific genes associated with pain processing in pain. These include genes for the transient receptor potential cation channel, subfamily V, member 1 (TRPV1); catechol-O-methyltransferase (COMT); and proprotein convertase gene 6 (PCSK6).33–35 There is limited data about therapy but evidence suggests that duloxetine (a serotonin and noradrenaline re-uptake inhibitor) has a modest but significant effect on pain in osteoarthritis of the knee.36
Illustrative case
A woman aged 65 years, who is a retired gardener, presents with a 5-year history of progressive mechanical knee pain and swelling. She has some night pain and morning stiffness of 15 minutes. Before your consultation, she had tried full-dose paracetamol, glucosamine sulfate, rose hip vital, fish oil, physiotherapy, conservative weight loss programs and ibuprofen (in doses up to 800 mg/day). None of the treatments has been particularly beneficial and she finds it hard to walk more than 100 metres or climb stairs.
She has a background history of lumbar spondylolisthesis, hypertension (well controlled on an angiotensin converting enzyme inhibitor and diuretic), hypercholesterolaemia (on atorvastatin), obesity (BMI 38 kg/m2) and smoking. There is a strong family history of rheumatoid arthritis: her grandmother, father and aunt had this condition.
Examination shows an overweight woman with a full range of movement in both knees. She has small effusions, crepitus on movement and some medial bony enlargement and tenderness. Her muscles seem a little weak but you are unsure if this is due to pain. Hip movements are normal.
Radiographs show grade 1 (mild) joint space narrowing. Basic blood tests, including erythrocyte sedimentation rate (ESR), CRP, creatine kinase (CK) and rheumatoid factor, are normal. You diagnose osteoarthritis of the knees and consider a number of initial management strategies.
You consider NSAIDs in therapeutic doses are risky given her antihypertensive regime. Stopping her atorvastatin for a month may improve muscle function and pain but is unlikely to help the knee inflammation. You decide to drain the fluid from her knees and give her intra-articular corticosteroid injections (eg. 80 mg methylprednisolone) into each knee. This gives her 10 weeks of relief so you repeat this regimen approximately every 3 months.
After 2 years, the injections stop helping so you decide that further injections are unlikely to be worthwhile. She asks what other options are available. You discuss surgically assisted weight loss and joint replacement but she is not interested in either option. She is interested in a trial of zoledronic acid reported in a recent publication so you refer her for a rheumatology opinion.
An MRI scan shows a large medial plateau lesion (Figure 1). Six months after receiving zoledronic acid, a repeat MRI scan shows total resolution of the bone marrow lesion (Figure 2). However, the patient says there has been little, if any, change in pain.
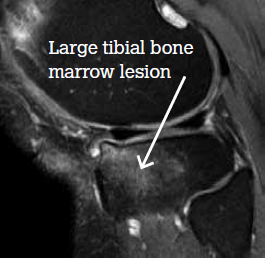
Figure 1. MRI scan at baseline
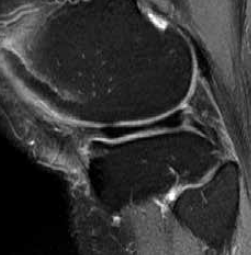
Figure 2. MRI scan 6 months later
You again discuss options and take the view that lap banding may be more appropriate than joint replacement in this case given the significant obesity, the relatively mild joint changes and the literature suggesting overweight people have less successful joint replacement outcomes. She agrees to have lap banding done and loses 36 kg over 18 months. Her pain score is now 2/10 and she is on no pain medication and doing all the activities she wants to do. You leave follow up open depending on progress.
Key points
- Osteoarthritis is an umbrella term for a number of processes that lead to pain and/or cartilage loss.
- It is clear that osteoarthritis is an active process rather than merely wear and tear.
- Treatment should be tailored to the individual as same size does not fit all.
- Targeting subchondral bone has the most potential to modify osteoarthritis given failure of most therapies aimed at cartilage.
Competing interests: Graeme Jones has board membership on Novartis, Roche, Amgen, Abbott, Merck Sharpe & Dohme, Bristol–Myer Squibb, UCB, Pfizer, Janssen and Servier, and has received honoraria for educational presentations from Servier and MSD. He has also received honoraria from Novartis, Roche, Amgen, Abbott, MSD, BMS, UCB, Pfizer, Janssen and Servier for consultancy and lectures.
Provenance and peer review: Commissioned; externally peer reviewed.