Definition
An aneurysm is an artery that has enlarged to greater than 1.5 times the expected diameter.1 In the infrarenal aorta, the threshold diameter is accepted as 3.0 cm in the Caucasian population. Anatomical reference terms for aortic segments are illustrated in Figure 1.
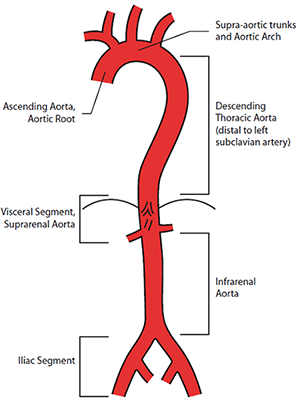
Figure 1. Anatomical reference terms for aortic segments
Image courtesy Cook Medical, Bloomington NH, USA
Abdominal aortic aneurysm
Prevalence and natural history
Abdominal aortic aneurysm (AAA) is rare in people aged less than 50 years, but prevalence then rises sharply with increasing age. Abdominal aortic aneurysm affects approximately 4–7% of men and 1–2% of women over the age of 65 years.2–5 Established risk factors for AAA include advancing age, male gender, smoking and family history (Table 1).2
Table 1. AAA risk factors
- Advancing age
- Male gender
- Smoking
- Family history
- Atherosclerosis
- Hypertension
- Hypercholesterolaemia
- Other vascular aneurysm
|
The burden of aneurysmal disease seems to be stable in Australia, with approximately 4600 hospital separations for non-ruptured and ruptured AAA each year between 1998 and 2008. Approximately 1000 deaths annually are attributed to aortic aneurysms.6
The natural history is ongoing expansion, with increased risk of rupture as the aneurysm enlarges (Table 2).7 Aneurysms <5.5 cm expand at an average rate of 2–3 mm each year, with larger aneurysms expanding more rapidly. Aneurysms >5.5 cm diameter in men, and >5.0 cm in women, are at significant risk of rupture and should be considered for repair unless major contraindications exist.
Table 2. 12 month AAA rupture risk by diameter
| AAA diameter (cm) | Rupture risk (%/year) |
|---|
| 3.0–3.9 |
0% |
| 4.0–4.9 |
1% |
| 5.0–5.9 |
1–10% |
| 6.0–6.9 |
10–22% |
| >7.0 |
30–50% |
Screening and surveillance
Currently no formal AAA screening guidelines or programs exist in Australia, unlike Sweden, the United Kingdom and the United States.8–11 Screening with ultrasound in men over the age of 65 years has been demonstrated to reduce aneurysm related mortality in four large trials, including one performed in Western Australia.2–5,12 Considerable variation exists between international screening protocols, with concerns over value and effectiveness.13,14 Cost effectiveness may be increased by screening those at particularly increased risk of AAA, such as men with a history of smoking, or patients with atherosclerotic risk factors or a family history of AAA. More rigorous risk scoring systems are being explored.15
First degree family members of patients with a diagnosed AAA are at higher lifetime risk of developing a AAA of up to 20%,16–18 however, the genetic mechanisms for this are unclear.19 Previous recommendations have included screening for men and women over 50 years of age with a family history of AAA.20
The majority of AAAs detected with screening are below the threshold for elective repair. The management of patients with small AAA most importantly includes cardiovascular risk management with lifestyle advice, smoking cessation, pharmacotherapy (anti-hypertensives, statins, beta-blockers), and ongoing aneurysm surveillance. Suggested surveillance intervals are listed in Table 3, although local protocols may vary based on accessibility to imaging, patient preference and logistical factors.
Table 3. Suggested AAA surveillance intervals
| AAA diameter (cm) | Surveillance interval (months) |
|---|
| 3.0–3.9 |
24 |
| 4.0–4.5 |
12 |
| 4.6–5.0 |
6 |
| >5.0 |
3 |
While pharmacological interventions such as doxycycline, beta-blockers, statins and angiotensin pathway inhibitors to reduce AAA growth and rupture have been promising in animal models, these benefits have not been consistently reproduced in human studies.21,22 Optimal cardiovascular risk factor management should include smoking cessation, a statin, antiplatelet and antihypertensive agents to improve life expectancy by reducing cardiovascular mortality. In addition to preventing aneurysm related mortality, screening for AAA will identify patients with small aneurysms who are at increased risk of cardiovascular events and who will benefit from cardiovascular risk management.
Many patients are under the misapprehension that a diagnosis of small AAA prohibits them from physical activity, or overseas travel. There is no evidence that physical activity leads to aneurysm rupture.
The role of imaging
A number of imaging modalities are available for the assessment of AAA. Ultrasound is a relatively cheap, non-invasive, widely available and reliable tool for detecting and measuring AAAs. It is the modality of choice for screening and surveillance.
Computed tomography angiography (CTA) is fast, reproducible and accurately depicts aneurysm morphology. It is the investigation of choice when considering potential surgical repair, and enables multiplanar analysis. Limitations include the need for high doses of intravenous iodinated contrast (potentially harmful in patients with renal impairment), and the use of ionising radiation.
Magnetic resonance angiography has limited utility in AAA. Digital subtraction angiography has largely been replaced by CTA for preoperative planning. It is now predominantly reserved for therapeutic purposes and is utilised during endovascular aneurysm repair (EVAR) to accurately position the stent graft before deployment.
In AAA surveillance imaging, the most relevant dimension is the maximum transverse diameter, ideally perpendicular to the line of flow. Aneurysm length is often reported, but is not relevant, except in planning for surgical repair. The presence of mural thrombus is also generally not relevant unless the aorta is critically stenosed or there is evidence of distal embolisation.
When to refer
At initial diagnosis, patients may be referred for general advice and counselling, and in many cases specialist vascular surgeons and units will be happy to coordinate regular surveillance imaging and review. Otherwise, patients should be referred for review when the aneurysm approaches the size threshold for surgery. Patients with a known AAA presenting with abdominal or back pain, syncope or tenderness over the aneurysm, require urgent assessment by a vascular surgeon for potential surgery.
The benefit of elective aneurysm repair is clear in low and average risk patients with large aneurysms (men >5.5 cm; women >5.0 cm). The optimal management of small AAAs (4.0–5.5 cm) has been clarified by a number of large randomised trials which demonstrate no long term survival benefit with open or endovascular repair.23–26 Even with low perioperative mortality (<1%) there is no survival benefit with elective repair, due to the very low incidence of rupture in small AAAs (<1%).
While decision making in AAA management can be complex due to patient age, comorbidities and aneurysm anatomy, it remains a balance between risk of rupture, operative mortality and life expectancy. Common indications for repair of AAAs are listed in Table 4.
Table 4. Indications for AAA repair
- Male with AAA >5.5 cm
- Female with AAA >5.0 cm
- Rapid growth >1.0 cm/year
- Symptomatic AAA (abdominal/back pain/tenderness, distal embolisation)
|
Repair of abdominal aortic aneurysm
Open repair
Due to the effectiveness of AAA repair, deaths related to rupture are potentially preventable. The first open AAA repair was performed in France by Charles Dubost in 1951.27 Since then, significant improvements in operative techniques, anaesthesia, and postoperative care have led to markedly lower morbidity and mortality from open AAA repair. Open repair of infrarenal AAA is illustrated in Figures 2a to 2c.
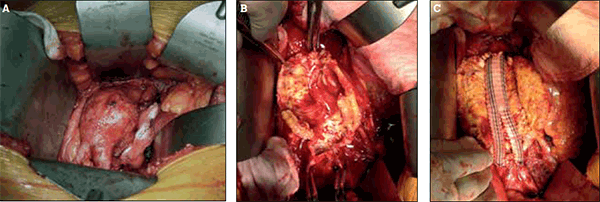
Figure 2. Open repair of infrarenal abdominal aortic aneurysm
A) Open exposure of an infrarenal aortic aneurysm via midline laparotomy; B) Intraoperative view of the opened aortic aneurysm sac; C) Bifurcated dacron graft (Vascutek Gelsoft™, Scotland, UK) sewn in place
The mortality associated with open elective repair in the literature varies from 1% in centres of excellence, to 8% in population studies.28 In the first 2 years of the Australasian Vascular Audit (2010–11), the perioperative mortality in 1302 non-ruptured open AAA repairs was 2.7%.29–31 The effectiveness of open AAA repair at preventing rupture and its long term durability are well established.32
Endovascular aneurysm repair
In 1990 in Buenos Aires, Parodi and colleagues performed the first EVAR.33 With rapid advances in techniques and technology, EVAR was embraced as a less invasive intervention for AAA.
Initially offered to patients deemed unfit to undergo open repair, it became apparent that EVAR was associated with significantly lower perioperative morbidity and mortality than open repair. Three randomised trials, DREAM, EVAR1 and OVER have demonstrated significantly lower operative mortality of EVAR compared to open repair (Table 5).34–36
Table 5. Perioperative mortality in randomised trials of EVAR vs open repair34–36
| Trial | EVAR | Open repair |
|---|
| EVAR1 |
2.3% |
6.0% |
| DREAM |
1.2% |
4.6% |
| OVER |
0.5% |
3.0% |
Not all AAAs are anatomically suitable for EVAR. An adequate sealing zone above and below the AAA, and adequate access vessels (common femoral and iliac) to accommodate the large stent graft delivery systems, are required for EVAR to be performed successfully. Endovascular repair of infrarenal AAA is illustrated in Figures 3a to 3d. Unfavourable anatomy for EVAR, such as a short, angulated aneurysm neck, severe iliac artery tortuosity, and calcification, increases the risk of complications and graft failure.
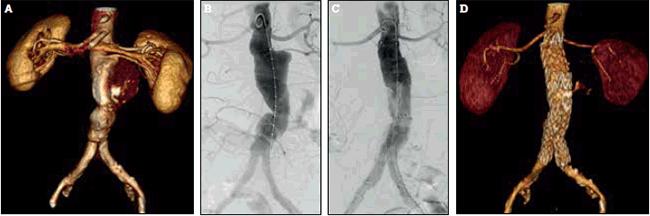
Figure 3. Endovascular repair of an infrarenal abdominal aortic aneurysm
A) CT angiogram reconstruction demonstrating an infrarenal abdominal aortic aneurysm; B) Intraoperative digital subtraction angiogram prior to stent-graft deployment; C) Intraoperative digital subtraction angiogram after stent-graft deployment (Medtronic Endurant®, Minneapolis MN, USA). Note that the left lower pole renal artery has been deliberately sacrificed to maximise sealing of the stent-graft; D) Postoperative CT angiogram reconstruction demonstrating the aorta and graft
Postoperative care
Open exposure of the aorta and aortic cross clamping predispose to postoperative cardiac, respiratory and renal complications (Table 6).28 Patients typically require 1–2 days in an intensive care unit and the average hospital length of stay is 5–7 days. Some patients will require inpatient rehabilitation before returning home.
Table 6. Risk of perioperative complications following open repair28
| Cardiac |
5.0% |
| Pulmonary |
4.2% |
| Renal |
1.7% |
| Sepsis |
0.7% |
| Stroke |
0.4% |
| Wound complications |
3.3% |
| Bowel obstruction or ischaemia |
2.0% |
| Amputation |
0.1% |
Complications related to the aortic repair are uncommon, but include graft infection 1%, aortoenteric fistula <1%, anastomotic pseudoaneurysm 1–2%, and limb occlusion 5%.28 Bowel obstruction and incisional hernia may also be long term sequelae of open repair.
Endovascular aneurysm repair can be performed under local or regional anaesthesia with reduced operative time, blood loss and pain compared to open repair. Intensive care unit admission is not routinely required and patients are typically discharged home in 2–3 days. The procedure can now be safely performed percutaneously, without the need for a large cut down onto the common femoral artery in the majority of cases.
The long term durability of EVAR is not as proven as open repair, and reintervention is required more frequently. This is most commonly due to the presence of an ‘endoleak’. Endoleak does not refer to a ‘leaking’ or ‘ruptured’ aneurysm, but to flow outside of the stent-graft but within the aneurysm sac. Endoleak has the potential to compromise the aneurysm repair and carries a small risk of rupture.37–39 Due to the risk of delayed complications with EVAR, lifelong surveillance is indicated. This is usually performed on a yearly basis, with a combination of plain abdominal X-ray, duplex ultrasound and CTA.
Ruptured abdominal aortic aneurysm
Ruptured AAA is a catastrophic event associated with an overall mortality of 80–90%.40 Rupture into the peritoneal cavity is usually rapidly fatal, whereas retroperitoneal rupture may transiently stabilise, providing a window of opportunity for lifesaving intervention. Patients should be transported to a vascular surgical centre immediately, and hypotensive resuscitation instituted to prevent excessive blood loss. Nevertheless, the majority of patients die before arrival at a surgical centre.
Mortality with open repair for ruptured AAA has stabilised at 30–40%. The major surgical advancement in the management of ruptured AAA has been the introduction of EVAR. Early results suggest improved survival with EVAR compared to open repair in ruptured AAA, however a number of randomised trials are currently underway to determine the true benefit of EVAR in ruptured AAA.
Other aortoiliac aneurysms
Isolated iliac artery aneurysms (Figures 4a and 4b) may be associated with AAA or occur independently. Rupture of these aneurysms is associated with high mortality, and intervention should be considered if they exceed 3.5 cm. Open repair may require ligation, and sometimes reconstruction, of the iliac bifurcation. Endovascular repair may involve embolisation of the internal iliac artery, except where branched iliac stent-grafts are suitable.
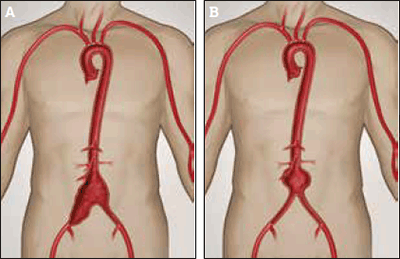
Figure 4a. Aortoiliac aneurysm involving the infrarenal aorta and right common iliac aneurysm
Figure 4b. Infarenal aortic aneurysm
Image courtesy Gore Medical, Flagstaff OH, USA
Thoracic aortic aneurysm (TAA, Figure 5) and thoracoabdominal aortic aneurysm (TAAA, Figure 6) are generally considered for repair at a maximal diameter exceeding 6.0 cm. All patients with TAA or TAAA should be referred to a vascular or cardiothoracic surgeon for assessment and consideration for repair. Given the high morbidity and mortality associated with open repair, and the availability of complex branched and fenestrated stent-graft designs, endovascular repair is now the preferred mode of treatment for descending thoracic and thoracoabdominal aortic aneurysms.
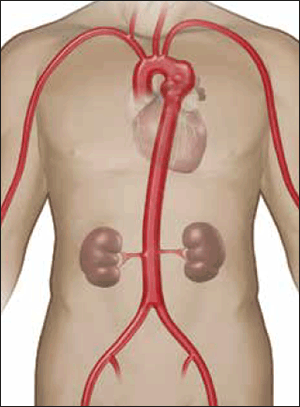
Figure 5. Descending thoracic aortic aneurysm located just distal to the left subclavian artery
Image courtesy Gore Medical, Flagstaff OH, USA
Aortic arch or ascending aortic aneurysm requires cardiac bypass for open reconstruction, and in most cases this is performed by a cardiothoracic surgery team, often in conjunction with a vascular surgeon.
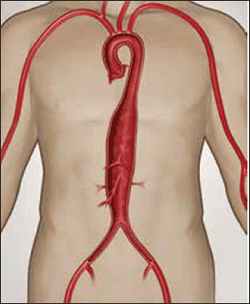
Figure 6. Crawford Type 3 thoracoabdominal aneurysm
Image courtesy Gore Medical, Flagstaff OH, USA
Aortic dissection
Aortic dissection is a condition of delamination of the aortic wall, leading to the development of a ‘double-barrel’ aorta, compromised luminal and side branch flow, and weakening of the aortic wall predisposing to aneurysmal degeneration. Figure 7 indicates a Stanford Type B aortic dissection, arising distal to the left subclavian artery. Unlike Type A dissections, which involve the aortic arch, Type B dissections are best managed with a combination of medical therapy and endovascular stent-grafting where necessary.
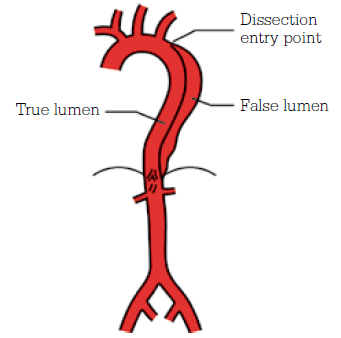
Figure 7. Stanford Type B aortic dissection
Image courtesy Cook Medical, Bloomington NH, USA
Patients may present with thoracic aortic ‘dissecting’ aneurysms, either due to acute or chronic degeneration. Acute dissection is often rapidly fatal, especially if there is retrograde dissection to the coronary arteries. Antegrade dissection can lead to renal, mesenteric, or lower limb ischaemia. Patients typically present with sudden onset interscapular pain, and if they survive the first 24 hours require aggressive blood pressure control and monitoring. Any suspicion of acute dissection requires immediate assessment in an emergency department with cardiovascular services, where the patient will be triaged based on the type of dissection.
Key points
- The prevalence of AAA sharply increases with increasing age.
- Rupture is associated with a high mortality.
- Screening men over the age of 65 years reduces aneurysm related mortality.
- Patients diagnosed with small AAA should have ongoing surveillance with ultrasound and cardiovascular risk factor modification, and may be referred to a vascular surgeon for counselling about management options.
- Patients with indications for AAA repair should be referred promptly to a vascular surgeon.
Competing interests: Jason Chuen has received payment for lectures from Medtronic and Cook Medical, and panel membership of Medtronic.
Provenance and peer review: Commissioned; externally peer reviewed.