The implications of antibiotic resistance for the future of healthcare are frightening. Quite apart from serious infections (such as pneumonia and sepsis), which are now managed routinely with antibiotics, we face huge challenges for much of our medical ‘high technology’ (such as joint replacement, chemotherapy and cardiac catheterisation), which is dependent on antibiotic ‘cover’ (prophylaxis). Risk benefit comparisons will change.
Of course the solution is, on the face of it, quite simple. Stop prescribing antibiotics. However, this is difficult to achieve for many reasons. At the core is the problem of the ‘failure of the commons’:2 resistance is a shared, delayed and remote problem while the patient’s wellbeing is ‘mine’, immediate and proximal.
Acute respiratory infections (ARIs) comprise 10% of primary care consultations in Australia and an antibiotic is prescribed in 58% of cases.3 For example, in acute otitis media (which accounts for 7% of all general practitioner consultations with children in Australia), antibiotics are prescribed for 82.3% of consultations,4 despite systematic reviews in the 1980s showing the benefit of antibiotics is very small. Extrapolating from overseas, Australians consume an estimated 1000 tonnes of antibiotics per year,5 most prescribed in primary care rather than through hospital or specialist care.
Prescribing antibiotics in primary care is associated with a doubling of the odds (OR 1.95, 95% CI: 1.1–3.5) of isolating antibiotic resistance commensal organisms 2 months later.6
Following publication of some seminal systematic reviews in the 1980s and 1990s, antibiotic use for these conditions began to fall and it looked as if with time the crisis would pass. However, for the past two decades, antibiotic use has plateaued at a level much higher than in northern European countries. This means that relying solely on interventions that minimise resistance by reducing antibiotic prescribing is risky and that a broader front of attack is necessary.
Both clinician and patient-related factors are important in antibiotic resistance. It is difficult to dissuade clinicians from overprescribing: they wish to support their patients who have an ARI, even when they believe that antibiotics are only minimally effective, perhaps because their attitude is ‘my patient wants me to do something’.7 However, patients with a sore throat who hope for antibiotics when attending a consultation actually often want symptom relief, which could be better provided by analgesics than antibiotics.8 Perceived patient expectations are also important. General practitioners are nearly three times more likely to prescribe antibiotics if they believe patients expect them.9 Other clinician-related factors include concern about diagnostic uncertainties in ARIs and missing important bacterial illnesses (such as pneumonia and meningitis) and downstream commercial incentives in a fee-for-service environment where consultations with prescribed antibiotics are easier, terminated more quickly and effectively, and result in patients being 40% more likely to re-consult for future ARIs.10
Patients (or parents) often have misperceptions about the benefits and harms of antibiotics. Some parents of children with acute otitis media assume that antibiotics are the best and only way to treat the illness, do not perceive antibiotics to have any harms, and do not entertain the idea of treatment that does not include antibiotics. Patients with an ARI often self-medicate with antibiotics that they (or a family member) already have at home, with little or no awareness of the individual and population harms of doing so – another contributor to resistance.11
We hypothesise that most clinicians and most patients do not understand that population antibiotic resistance can be reversed – as data from Iceland show.12
Few patients or clinicians know about person-to-person transmission of resistance genes. Bacteria from different species commonly exchange genetic material, including ‘resistance’ genes. As humans harbour about 1014 bacteria (ie. 10 for every human cell), antibiotics will affect nonpathogenic bacteria (commensals) as much as pathogenic. Therefore, there is a huge opportunity for resistance genes to colonise other people using normal contamination transmissions: in other words, resistance can be transmitted in the same way as infection (Figure 1). Therefore, interventions that reduce acute respiratory infection transmission (known to be effective13) should also reduce resistance gene transmission.
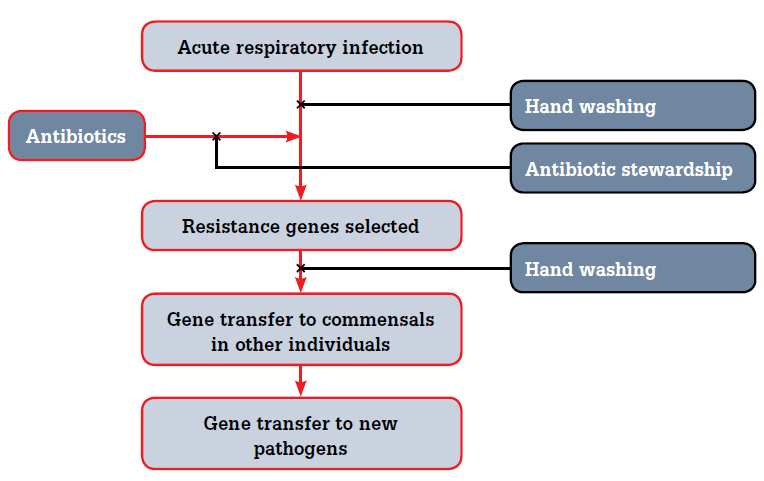
Figure 1. Causes of antibiotic resistance
It is timely therefore, that the award of National Health and Medical Research Council multidisciplinary Centre for Research Excellence funding has provided the opportunity for a group of us to generate new knowledge to support the superb efforts of the National Prescribing Service, which has been addressing antibiotic and other prescribing challenges. These areas for new knowledge generation are outlined in Table 1.
Table 1. Areas for new knowledge generation
| Thinking more critically about harms and benefits of antibiotics for acute respiratory infections. We have heard much about the paucity of benefits of antibiotics, but perhaps insufficient about the harms (the thrush, rashes, gastrointestinal upsets as well as more serious adverse effects) which need to be introduced into the riskbenefit equation |
| Adopting higher rates of hand washing to reduce person-to-person infection with both acute respiratory infections and resistance genes (carried by normal commensals) |
| Addressing the labelling of antibiotic packaging better: we have packet sizes that allow for unused doses or new packets that have to be broached |
| Increasing the uptake of delayed prescribing in Australia. This 'safety net' works well at reducing antibiotic consumption overseas, but has never been trialled in Australia |
| Addressing the problem as resistance genes rather than infectious agents. The issue here is that resistance can be transmitted via gene transfer from pathogens to commensals and back again. How resistance is spread through the community is poorly understood, although we know that it is generated easily enough with antibiotic use |
| Modelling all these approaches to estimate which ones are likely to yield the best returns in reducing antibiotic resistance |
We will be seeking practices interested in joining us in undertaking research projects on these issues. For more information or to express your interest in participating in this research please email CREMARA@bond.edu.au.
Conflict of interest: none declared.