Case study 1
A woman, 37 years of age, presented to the emergency department with 4 days of productive cough, shortness of breath, chest tightness, fevers and an episode of haemoptysis. She had been seen by her general practitioner and treated with amoxycillin 875 mg with clavulanic acid 125 mg and azithromycin 500 g for presumed lower respiratory tract infection (LRTI). Her past medical history was unremarkable except for laparoscopic adjustable gastric banding (LAGB) in 2011; the band was further inflated 5 days before her presentation to hospital. In retrospect, she reported experiencing nausea and vomiting since that time.
On examination, the patient was tachypnoeic and mildly hypoxaemic with an oxygen saturation of 97% on 6 L/min oxygen via a Hudson mask. She was otherwise haemodynamically stable and afebrile. On examination of her respiratory system, there was widespread wheeze and bilateral crepitations. Her inflammatory markers were elevated. Consolidation in both lower zones was noted on chest X-ray (Figure 1).
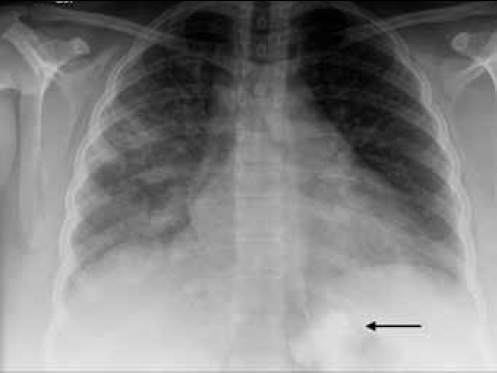
Figure 1. Chest X-ray of Case 1 showing bilateral lower zone increased air space opacities. Note the correct position of the LAGB inferior to the diaphragm (arrow)
The patient was discharged on antibiotic therapy and her surgeon deflated the LAGB the following day. Her respiratory symptoms had fully resolved after 1 week.
Case study 2
A woman, 61 years of age, presented to the emergency department with a 7 day history of productive cough, fever, rigors and small amounts of haemoptysis. She had been seen by her GP 3 days previously and was prescribed oral antibiotics for a presumed community acquired pneumonia. In retrospect, she admitted to daily vomiting for several weeks.
On the day of presentation, she experienced an episode of central chest pain that lasted for half an hour, which was associated with diaphoresis. Her medical background was unremarkable except for hypercholesterolaemia and LAGB in 2009. A couple of months before her presentation, her gastric band was inflated because her weight loss had plateaued and had been partially deflated again due to intractable nausea and vomiting.
On examination, the patient was afebrile and haemodynamically stable, with unremarkable respiratory and cardiovascular examinations. Electrocardiogram was not suggestive of an acute ischaemic event and her cardiac enzymes were within normal range. As pulmonary embolism was suspected on presentation to the emergency department, in addition to a chest X-ray, a computed tomography pulmonary angiogram (CTPA) was performed. The chest X-ray showed an air fluid level in the mediastinum consistent with oesophageal dilatation (Figure 2). The CTPA excluded pulmonary embolism but also revealed oesophageal dilatation and retention of gastric contents proximal to the gastric band (Figure 3).
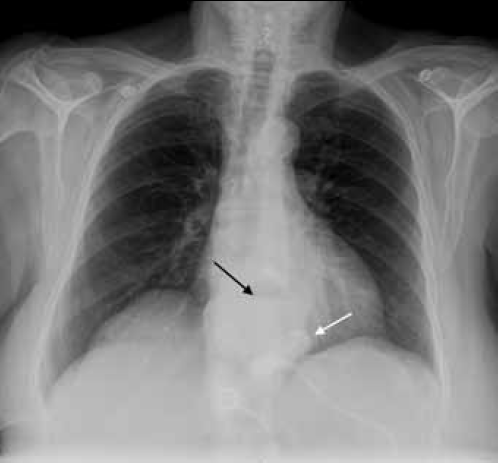
Figure 2. Chest X-ray of Case 2 showing an air fluid level in the mediastinum (black arrow) consistent with oesophageal dilatation. Note that the LAGB is visible above the level of diaphragm (white arrow), suggestive of slippage into the inferior mediastinum. This is confirmed on Figure 3
Her symptoms resolved completely within 24 hours of deflation of her LAGB and she was discharged home on a course of oral antibiotics. The patient was reviewed by her surgeon 3 days later and a revision of the band was planned.
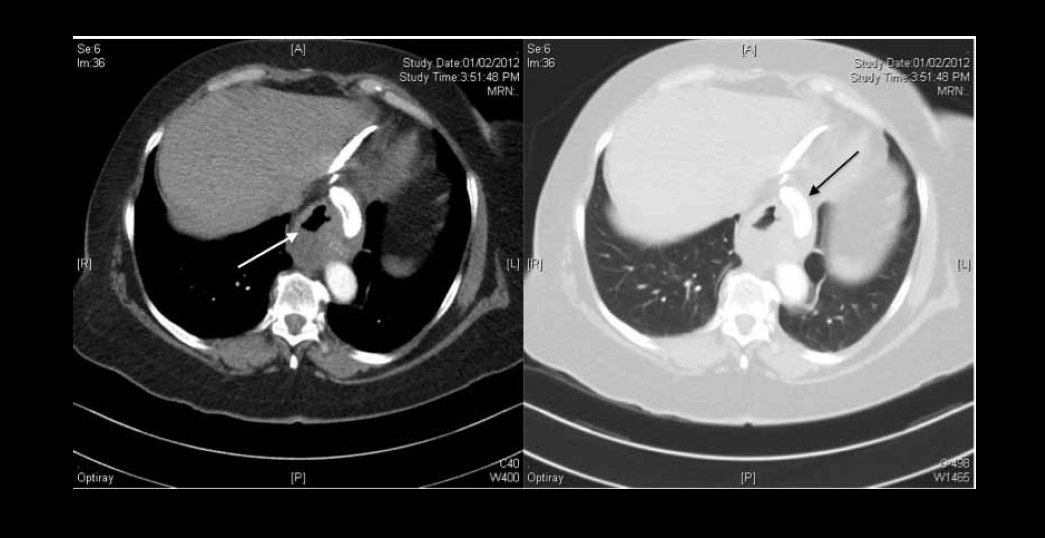
Figure 3. CT scan of Case 2 confirming clear lung fields and dilated oesophagus (white arrow). The LAGB has slipped into the inferior mediastinum (black arrow)
Case study 3
A man, 55 years of age, presented to the emergency department with an acute episode of chest pain. He had been suffering from intermittent haemoptysis, cough, fevers and vomiting after meals for approximately 1 year before this presentation. He had been treated five times with antibiotics by his GP for suspected pneumonia in the preceding 3 months. His past medical history was significant for type 2 diabetes mellitus, gastro-oesophageal reflux disease, cholecystectomy and LABG in 2010, which was inflated most recently in mid 2011.
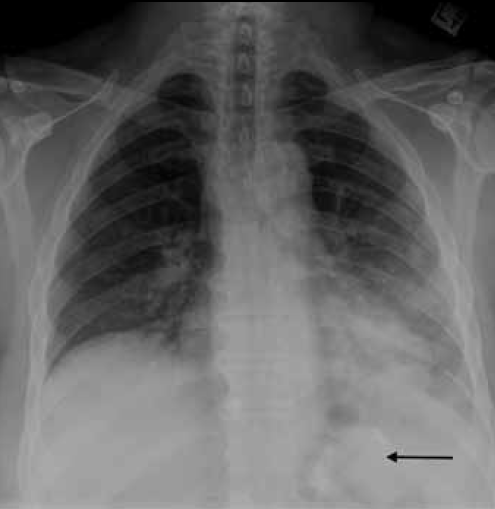
Figure 4. Chest X-ray of Case 3 showing left lower zone air space opacity. Note the correct position of the LAGB inferior to the diaphragm (arrow)
On examination, the patient was haemodynamically stable and afebrile. He had left basal inspiratory crepitations on chest auscultation as well as epigastric tenderness on abdominal examination. His inflammatory markers were elevated and his chest X-ray revealed a left basal opacity consistent with consolidation (Figure 4). A CTPA was performed to investigate for pulmonary embolism (Figure 5). This showed diffuse ground glass changes in the left lung as well as oesophageal dilatation. His symptoms resolved completely following deflation of his LAGB and he was discharged on a course of oral antibiotics.
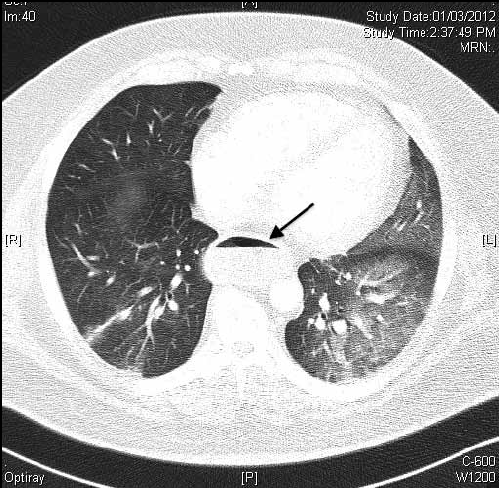
Figure 5. CT scan of Case 3 showing bilateral air space opacities and oesophageal dilatation with air fluid level (arrow)
With the current epidemic of obesity in Australia,1 bariatric surgery has become increasingly accessible for patients with a body mass index above 35 kg/m². The number of bariatric surgeries in Australia has risen from approximately 500 in 1998 to 17 000 in 2007–08.2
Laparoscopic adjustable gastric banding (LAGB) is considered the safest of the bariatric surgical procedures, with a low mortality rate of 0.05%.3 It is relatively simple to perform by appropriately trained laparoscopic surgeons, can be reversed if the there are complications related to the tightness of the band, and is of relatively low cost to the health system.
Commonly described complications include:4
- early complications: acute stomal obstruction, band infection, gastric perforation, haemorrhage, delayed gastric emptying and pulmonary embolism
- late complications: band erosion, band slippage or prolapse, port or tubing malfunction, leakage, oesophageal dilatation and oesophagitis.
In addition to these complications, medium term respiratory complications can occur. However, reporting of respiratory complications is limited to sporadic case reports.5–7 Although aspiration pneumonia is mentioned as a possible late complication of oesophageal dilatation in the context of LAGB, the emphasis has been on band-related complications (eg. slippage, leakage and malfunction).4
We report three cases of late respiratory complications in patients with a history of LAGB. In all three cases, the gastric band was inserted more than 12 months before presentation. However, all had respiratory symptoms within 6 weeks of further tightening of the band. All presented with productive cough, haemoptysis, dyspnoea and fever and in retrospect reported gastro-oesophageal symptoms of nausea and vomiting. Central chest pain was also a frequent complaint and in two cases was the reason for hospital admission and further investigations. In each case, a simple chest X-ray was the most useful imaging modality for identifying the presence of the LAGB device and determining its position. Symptoms resolved in all three patients following deflation of the LAGB. The patient in Case study 2 required revision of her LAGB due to slippage.
Oesophageal dilatation proximal to the band device has been observed in as many as 10% of patients.8 It may develop when the band is excessively inflated or in the setting of excessive amounts of food intake. This may result in recurrent aspiration of gastric contents into the lower respiratory tract.
Summary
A history of weight loss surgery should be actively sought in all patients presenting with symptoms of reflux and aspiration. Central chest pain in this clinical context would be explained by oesophageal spasm and/or oesophagitis rather than pulmonary embolism and may not require a CTPA. A simple chest X-ray is the initial investigation of choice and may obviate the need for further radiation exposure and hospital admission.
Conflict of interest: Tajalli Saghaie has received financial support from Boehringer Ingleheim to attend meetings. Lucy Morgan has received payment from GlaxoSmithKline, AstraZeneca, Pfizer and Novartis for lectures.