Epidemiology and aetiology
Acute rheumatic fever predominantly affects Aboriginal and Torres Strait Islander children aged 5–14 years living in regional and remote areas of central and north Australia. It has an estimated incidence of 250–350 per 100 000.2 However, little is known about the epidemiology of ARF and RHD in southern regions of Australia, despite the fact that an estimated 57% of the Aboriginal and Torres Strait Islander population live in these regions.3,4
Group A streptococcus (S. pyogenes) is the most common cause of acute bacterial pharyngitis (Figure 1) – a precursor to ARF – and is primarily spread by person-to-person contact. However, among Aboriginal and Torres Strait Islander populations in the 'top end' of Australia, streptococcal pyoderma (Figure 2) is the main cause of superficial GAS infection and rates of pharyngitis are low.5 Group C and G beta-haemolytic streptococcal pharyngitis has been implicated as a cause of poststreptococcal complications in this region, leading some researchers to suggest that use of selective testing methods for S. pyogenes to provide evidence of a preceding streptococcal infection may result in some cases being missed.6 Furthermore, it is hypothesised that streptococcal pyoderma may protect against streptococcal pharyngitis, thereby contributing to its lower prevalence in Aboriginal communities where there are high levels of skin infection with GAS.5 Importantly, the role of GAS skin infection as a causal factor in the development of ARF is the subject of ongoing debate7,8 and the lack of streptococcal pharyngitis documentation in these populations means uncertainty about the actual prevalence of preceding throat infection persists.5
Acute rheumatic fever occurs 10 days to 6 weeks after an episode of streptococcal infection. A definite complaint of a previous sore throat may or may not be present in the history. Acute rheumatic fever manifests as a nonsuppurative inflammatory process affecting cardiac tissue (Figure 3), joints, subcutaneous tissue and the central nervous system. The underlying disease process remains unclear, but a failure of the immune response to differentiate epitopes of the streptococcal pathogen from host cells and host susceptibility are both thought to be important factors.9
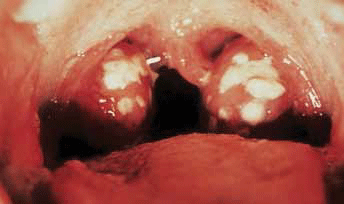
Figure 1. Acute streptococcal pharyngitis Image © Elsevier 2003. Reproduced with permission. Forbes CD, Jackson, WF. Acute streptococcal pharyngitis. Published in Color Atlas and Text of Clinical Medicine, 3rd edn
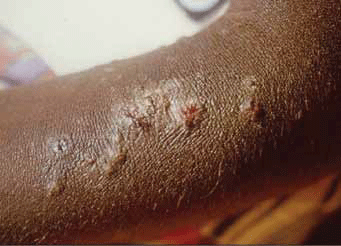
Figure 2. Streptococcal pyoderma seen in an Aboriginal child Image reproduced with permission Menzies School of Health Research
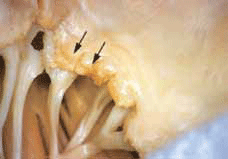
Figure 3. Acute rheumatic mitral valvulitis superimposed on chronic RHD Image © Elsevier 2005. Reproduced with permission Kumar et al. Robbins and Cotran Pathologic Basis of Disease, 7th edn
Diagnostic criteria
Initially described in 1944 by Jones,10 the diagnostic criteria for ARF separate the clinical features into major and minor manifestations – major manifestations being more specific for the disease. The Jones criteria were last revised in 1992 to increase their specificity for ARF because the incidence in developed nations had decreased significantly. In 2006, the National Heart Foundation of Australia (NHFA) and the Cardiac Society of Australia and New Zealand (CSANZ) developed diagnostic and treatment guidelines for use in Australia, allowing for increased sensitivity among identified high risk groups2 (Table 1).
High risk refers to groups with high rates of ARF, such as Aboriginal and Torres Strait Islander communities living in rural or remote areas or in disadvantaged suburban areas. Sydenham (rheumatic) chorea is present in roughly 25% of Aboriginal Australians (especially female adolescents) with ARF.11 As part of the major manifestations, Sydenham chorea is sufficient to diagnose ARF without evidence of previous S. pyogenes infection, provided other causes of chorea have been excluded.2 The NHFA criteria consider some less specific symptoms, such as polyarthralgia and aseptic monoarthritis, to be major manifestations in high risk groups (Table 1).
Evidence of preceding GAS infection can be gathered from serum streptococcal antibody titres. However, standard laboratory reporting uses upper limits of normal from adult populations and therefore does not reflect the normal titres in children. Table 2 lists upper limits of normal derived from non-Aboriginal and Torres Strait Islander children with no recent history of streptococcal infection.12
High risk groupsAll other groups
Table 1. Australian guidelines for the diagnosis of acute rheumatic fever2
| Initial episode of ARF |
Two major or one major and two minor manifestations
plus
Evidence of a preceding Group A streptococcus infection |
Recurrent attack of ARF
past ARF or RHD) |
Two major or one major and two minor or three minor manifestations
plus
Evidence of a preceding Group A streptococcus infection |
| Major manifestations |
Carditis (including subclinical evidence of rheumatic valve disease on echocardiogram)
Polyarthritis or aseptic mono-arthritis or polyarthralgia
Sydenham chorea
Erythema marginatum
Subcutaneous nodules |
Carditis (including subclinical evidence of rheumatic valve disease on echocardiogram)
Polyarthritis
Sydenham chorea
Erythema marginatum
Subcutaneous nodules |
| Minor manifestations |
Fever
ESR ≥30 mm/hr or CPR ≥30 mg/L
Prolonged P-R interval on ECG |
Fever
Polyarthralgia or aseptic mono-arthritis
ESR ≥30 mm/hr or CPR ≥30 mg/L
Prolonged P-R interval on ECG |
ESR = erythrocyte sedimentation rate; CRP = c-reaction protein; ECG = electrogardiograph
Reproduced with permission Diagnosis and Management of Acute Rheumatic Fever and Rheumatic Heart Disease in Australia. An evidence based review. © National Heart Foundation of Australia, 2006 |
Community occurrences and the role of the GP
In a recent study we found that during an 8 year period (2001–2008) an average of three children aged <15 years were admitted each year with ARF to the Children's Hospital at Westmead (CHW), a major tertiary paediatric centre in New South Wales.1 Seventy-seven percent of these children lived in suburban Sydney and over one-third had presented to their general practitioner first. Most were referred promptly to hospital, but in some cases delays occurred, eg. when children were initially diagnosed with a viral illness or diagnosed with a rheumatologic disorder and referred to a rheumatology service. Our results suggest that in some cases primary health physicians had a low index of suspicion for ARF, despite the presence of major manifestations of ARF. Almost half of the children identified as Aboriginal and Torres Strait Islander or Pacific Islander. In most cases, children presented only after they had experienced symptoms for a considerable period of time, perhaps reflecting lack of community awareness of the potentially serious consequences of symptoms of ARF, cultural preferences or difficulties in accessing medical care.
General practitioners are integral to ensuring early diagnosis and early treatment of ARF to minimise heart valve damage and progression to RHD. Clinical features raising a high suspicion of ARF are shown in Table 3. Work-up and management of suspected ARF is shown in Table 4. Following diagnosis of ARF, appropriate patient education and administration of secondary prophylaxis with benzathine penicillin G can prevent or decrease recurrences of ARF. An integral component of efficient and effective secondary prophylaxis is the establishment of local and centralised ARF/RHD registers.2,13
Following confirmation of ARF diagnosis, intramuscular (IM) benzathine penicillin G injections must be given every 3–4 weeks for a minimum of 10 years as secondary prophylaxis. This places a considerable burden on families and patients and is particularly challenging if the family does not have easy access to medical care. Adherence to the regimen is a major consideration, particularly if the patient finds the injections painful or believes them unnecessary. Patient education regarding the recurrent nature of ARF and the serious potential long term consequences of the disease is essential. Coordinated community care is central to maintaining adherence to prophylaxis.
If benzathine penicillin G injections are refused, oral penicillin V twice daily can be used as an alternative secondary prevention measure. However, a systematic review indicates that IM benzathine penicillin G is superior to oral penicillin V for reducing recurrent streptococcal pharyngitis and ARF.14 Oral penicillin (250 mg) is associated with poorer adherence and is more likely to result in variable serum penicillin concentrations, which may not reach optimal therapeutic levels.15 If the decision is made to use oral penicillin, patients and their families must be informed that this is not the optimal regimen, warned of the risks of missing doses and closely monitored to ensure adherence. In settings of high risk for ARF recurrence, every effort should be made to encourage the use of benzathine penicillin G rather than oral penicillin.
Ensuring adherence to prophylaxis is a significant challenge for primary care physicians caring for children living in remote regions and often within highly mobile populations. Automated reminder systems and better coordination and liaison among primary health services and community health services may improve rates of delivery and adherence, although this has not yet been proven. Control registers, although not currently established in some states, aim to improve long term coordination of care for patients with ARF. These registers are available in the Northern Territory, Western Australia and Queensland.
Table 2. Upper limits of normal12 for serum streptococcal antibody titres derived from 66 non-Aboriginal and Torres Strait Islander children, with no history of recent streptococcal infection
| | 80% upper limit of normal (IU/mL) |
|---|
| Age (years) | Anti-streptolysin-O titre (ASO) | Anti-deoxyribonuclease-B (anti-DNase-B) titre |
|---|
| 1–4 |
170 |
366 |
| 5–14 |
276 |
499 |
| 15–24 |
238 |
473 |
| 25–35 |
177 |
390 |
| ≥35 |
127 |
265 |
Table 3. Clinical features raising a high suspicion of acute rheumatic fever2
Symptoms and
history |
Sore throat in the previous 2–6 weeks
Fever
Lethargy and myalgia
Arthralgia (migrating arthralgia that is asymmetrical in pattern
and affects the large joints)
Indigenous Australians or Pacific Islanders* living in crowded
housing conditions |
| Examination |
Migratory polyarthritis† (extremely painful, affecting large joints)
Sydenham chorea‡ (in 25% of Indigenous Australians with ARF,
especially adolescent females)
Jerky movements of hands, feet, tongue and face
Carditis (apical pansystolic murmur or early diastolic murmur at
base of heart)
Subcutaneous nodules¥ (strong association with carditis)
Erythema marginatum§ (bright pink macules or papules on
extremities or trunk)
Fever ≥38.0°C |
Laboratory
markers |
Positive ASO and anti-DNase-B titres
Raised CRP ≥30 mg/L and ESR ≥30 mm/hr
Transient prolongation of P-R interval above 0.16–0.18 seconds
(more likely if returning to normal within 1–2 months) |
* Significant ethnic group in our urban NSW study
† Mono-arthritis may be a recurrent presenting feature in high risk populations
‡ Echocardiography is essential for all patients with chorea
¥Rare but highly specific manifestation of ARF
§ Highly specific for ARF but rare and difficult to detect in Indigenous Australian
patients
Ø Upper limit of normal varies with age group [see Table 2] |
Case study
Bill, an Aboriginal boy aged 13 years, lives in a small town in regional New South Wales. He presented to a local GP – accompanied by his grandmother – complaining of lethargy and pain in his knees and ankles. He had intermittent fever and his grandmother said he had been unwell for a 'long time'. The GP ordered blood tests, which showed leukocytosis (with an otherwise normal full blood count) and raised inflammatory markers. The autonuclear antibody (ANA) test was negative.
Six months later, Bill saw another GP in his home town for similar pains and fevers. This GP referred him to a paediatrician in a regional centre. Two friends of the family, who care for him most weekends, accompanied Bill. They said they had been concerned about his health for a year. The doctor noted that Bill shared a house with 20 other people. On examination, Bill appeared unwell and had a soft systolic murmur in the mitral area.
The paediatrician discounted the diagnosis of acute Ross River fever and expressed the possibility of acute rheumatic fever. A summary of Bill's key investigation findings is shown in Table 5.
Bill's paediatrician referred him for review in the cardiology department at Children's Hospital at Westmead. When Bill was seen at the hospital the following month he had pain in his right ankle and complained of occasional fever. The cardiologist administered 15 mg lisinopril and a starting dose of IM benzathine penicillin G. Bill was discharged 3 days later on a regimen of lisinopril 20 mg/day and three weekly benzathine penicillin G injections to be administered by the community nurse in Bill's hometown.
On review by the cardiologist 2 months later, Bill admitted to taking lisinopril only intermittently. However, he reported that his exercise tolerance was improved when he took his medication. The cardiologist noted on repeat echocardiogram that the degree of mitral incompetence had improved, but stressed that regular follow up was essential and strongly encouraged Bill to continue to take the lisinopril. The community nurse reported that threeweekly penicillin had been administered.
Table 4. Work-up and management of suspected acute rheumatic fever2
| Investigations |
Throat swab and culture for Group A streptococcus
Blood cultures if febrile
White cell count, ESR, CRP and ASO and anti-DNase-B titres
ECG
Chest X-ray if clinical evidence of carditis |
Investigations
for differential
diagnoses |
Repeat blood cultures if infective endocarditis suspected
Joint aspiration for septic arthritis
Serum copper, ceruloplasmin or antinuclear antibodies for
investigation of choreiform movements
Serology for arbovirus or autoimmune arthritis |
| Management |
Admit to hospital
Paracetamol (± codeine) for fever and joint pain
Single dose of IM benzathine penicillin G or 10 days of oral
penicillin V
Arthritis can be treated with aspirin or naproxen
Sydenham chorea requires no treatment in most cases
(carbamazepine and valproate if required)
Carditis requires bed rest and urgent echocardiogram. Diuretics
or fluid restriction may be necessary. Consider ACEI and/or
glucocorticoids for severe carditis |
Case discussion
It is likely that Bill had ARF in April when he first saw his GP. Important clues supporting the diagnosis of ARF include his Aboriginality and his social circumstances, including living in an overcrowded home. Appropriate management at this time would have been to confirm the diagnosis of ARF and prescribe aspirin for Bill's arthritis and IM benzathine penicillin G. It is possible Bill had had a recurrent episode of ARF leading to valvular compromise by the time he saw the paediatrician. This recurrence could have been prevented by the prompt administration of secondary prophylaxis. This case highlights the importance of educating patients about the consequences of recurrent ARF and involving family, friends and community carers in improving adherence to prophylactic treatment.
Key points
- ARF persists among children in rural and remote settings but also among those living in disadvantaged urban areas.
- Aboriginal and Torres Strait Islander and Pacific Islander children are over represented among children with ARF but are not the only ethnic group to suffer from this disease.
- Application of the NHFA guidelines for diagnosis in appropriate (including high risk) settings may help generate a high index of suspicion for ARF.
- Early detection of ARF and implementation of appropriate secondary prophylaxis regimens are essential to lower the rates of recurrent ARF and subsequent development of RHD.
- Improved awareness of ARF and better access to educational materials is needed among healthcare providers and vulnerable communities.
- Coordinated RHD control programs may be an efficient and effective way to improve benzathine penicillin G adherence and clinical care for the long term prevention of RHD.
Table 5. Bill's key investigation findings
| Investigation | Result |
|---|
| Full blood count |
Mild microcytic, hypochromic anaemia, low ferritin |
| Anti-streptolysin-O titre |
400 IU/mL (normal <320 IU/mL) |
| ESR |
54 mm/hr |
| CRP |
211 mg/L |
| Ross River virus |
IgM-/IgG+ |
| Chest X-ray |
Cardiomegaly
Clear lung fields |
| ECG |
Normal |
| Echocardiogram |
Moderately dilated left ventricle
Moderately dilated left atrium
Moderate mitral regurgitation
Mild aortic regurgitation, ? bicuspid aortic valve |
Further reading
Note: new guidelines published by RHD Australia and NHFA will have up-to-date recommendations on the diagnosis, management and secondary prevention of AF/RHD. They are due for release in 2012.
Conflict of interest: none declared.
Acknowledgements
The activities of the Australian Paediatric Surveillance Unit are supported by the Australian Government Department of Health and Ageing; the NHMRC: Enabling Grant No. 402784 and NHMRC Practitioner Fellowship No. 457084 (Elizabeth Elliott); The National Heart Foundation; The Discipline of Paediatrics and Child Health, Sydney Medical School, University of Sydney; and the Royal Australasian College of Physicians. Michael Smith was supported by the Summer Research Scholarship Programme, Sydney Medical School, University of Sydney.