When should HbA1c be ordered?
HbA1c reflects average glycaemia over the preceding 6–8 weeks. The test is subsidised by Medicare up to four times in a 12 month period.1 In some patients, HbA1c may be measured more frequently than 3 monthly to closely monitor glycaemic control (eg. in pregnancy when up to six tests in a 12 month period can be subsidised).1
The Service Incentive Program for diabetes care requires at least one HbA1c measurement per year. It is suggested that HbA1c is done every 6 months if meeting target, or every 3 months if targets are not being met or if therapy has changed.2
Self blood glucose monitoring (BGM) and HbA1c complement each other: BGM informs the patient about blood glucose at any particular time (eg. when the patient feels hypoglycaemic) and informs the patient and doctor about the glycaemic pattern over the 24 hour cycle and guides the timing and level of lifestyle intervention and hypoglycaemic therapy.
What do I tell my patient?
HbA1c is tested using venous blood, taken at any time of day and without any preparation such as fasting. In the paediatric setting, a finger-prick capillary sample can be used. A Medicare rebate is available for the test. Patients can usually understand that the HbA1c reflects the long term overall control of blood glucose, whereas self BGM measures the blood glucose at one particular time on one particular day. Patients can get to know what their target HbA1c level is and the reason for targets (including the increased risk of diabetic complications with higher levels of HbA1c).
How does the test work?
HbA1c is formed by a nonenzymic chemical reaction between glucose and the amino group of the N-terminal valine of the haemoglobin beta chain. Other forms of glycosylated haemoglobin are also formed by glycosylation on the alpha and beta chains (Figure 1). The rate of glycosylation depends on the blood glucose level (BGL) and HbA1c accumulates in the red cell during its circulation in the body (approximately 120 days) – newly formed red cells have no HbA1c and those about to be removed contain the most. The HbA1c assay measures the average percentage of haemoglobin, ie. HbA1c over the entire red cell population and has been found to mainly reflect the preceding 6–8 weeks. Glycosylation changes the electric charge and structure of the haemoglobin molecule and the three principles commonly used to separate HbA1c from the other members of the haemoglobin family are based on these changes (Table 1). There are many different assay systems, but all have been standardised to report the same numerical values as those in pivotal trials,3,4 which establish the rate of microvascular complication development and progression at different levels of HbA1c.
Table 1. Assays for HbA1c
| Methods | Analysis principle | HbA1c specificity |
|---|
| Ion exchange chromatography (HPLC) |
Slight changes in the isoelectric point |
Specific for HbA1c |
| Boronate affinity resin |
Structural differences cause binding to the resin |
All glycated haemoglobin (Figure 1) |
| Immunoassay |
Antibody to the changed structure of the N-terminal beta chain |
Varying specificity for HbA1c |
What do the results mean?
The HbA1c reflects the average glycaemic exposure that changes the risk of microvascular complications (retinopathy, nephropathy and neuropathy) increasing risk by approximately 30% for each 1% increase in the HbA1c level.3,4 The relationship between the onset and progression of microvascular complications is curvilinear, flattening as the HbA1c percentage decreases.
The general HbA1c target in people with type 2 diabetes is ≤7%, with diabetes treatment adjustment considered when HbA1c is above this level.5 However, individual glycaemic targets should be set, considering the potential harmful effects of optimising blood glucose in people with type 2 diabetes – lower targets for those who have most to gain and least to lose from improved glycaemic control (eg. women with gestational diabetes) and higher targets for those with least to gain and most to lose (eg. frail patients with limited lifespan).5
Changes in the level of HbA1c reflect changes in average glycaemia and the effect of hypoglycaemic intervention. If the assays are performed by the same laboratory using the same methods, the total variability of the HbA1c should be 8% or less. A change should be at least 16% to be considered to reflect a biological change (a 'signal') rather than reflecting the variability of the HbA1c measurement process (the 'noise').6
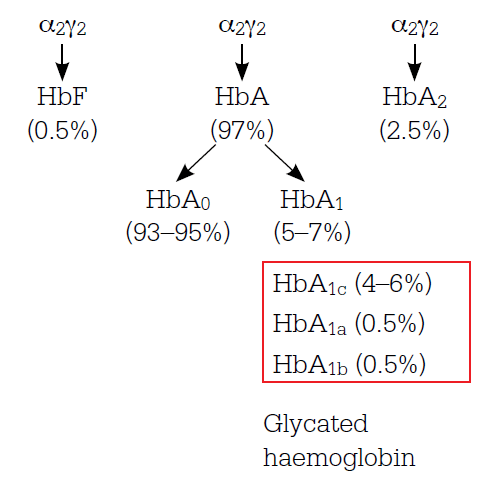
Figure 1. Haemoglobin components in adults without diabetes
Note: Haemoglobin contains four protein chains which interlock. Adult haemoglobin (HbA) is the main form of haemoglobin but small amounts of fetal haemoglobin (HbF) and haemoglobin A2 also occur. The glycated haemoglobins include haemoglobin A1a, b and c
What won't the results tell you?
Interpretation of results is limited by factors that affect the relationship between HbA1c and average overall glycaemia (Table 2). Furthermore, HbA1c does not reflect the swings in blood glucose, which can be as clinically important as overall glycaemia. Patients could have the same HbA1c but with frequent episodes of hypo- and hyperglycaemia or with stable glycaemia. As noted earlier, HbA1c testing and self BGM are complementary and provide information about different aspects of glycaemic control. The level of HbA1c is also affected by some pathophysiological conditions and some laboratory factors7 (Table 2). The shorter/ longer red cell survival the lower/higher the HbA1c will be for any level of average glycaemia. Changes in the glycation process increase/decrease glycation at any BGL and will increase/decrease HbA1c at any level of average blood glucose. Different forms of haemoglobin interact with the laboratory re-agents and have variable effects on the relationship between HbA1c and average blood glucose. The different HbA1c assays may be affected by a wide range of factors including clinical conditions, medications and haemoglobinopathies.
When HbA1c does not reliably assess average glycaemia (Table 2), alternate ways of assessing glycaemic control include self BGM and measuring other glycosylated proteins (such as fructosamine).
Table 2. Factors influencing HbA1c results7
| Factor | HbA1c result |
|---|
| Increased | Decreased | Variable changes |
|---|
Red cell survival
Erythropoiesis |
Iron deficiency
Vitamin B12 deficiency
Renal impairment |
Iron administration
Vitamin B12 administration
Erythropoietin treatment
Reticulocytosis
Chronic liver disease |
|
| Erythrocyte destruction |
Splenectomy |
Haemoglobinopathies
Splenomegaly
Rheumatoid arthritis
Medications (eg. antiretrovirals,
dapsone) |
|
| Glycosylation rate |
Vitamin C or E deficiency
Aspirin ingestion
Some haemoglobinopathies
Increased red blood cell pH |
Alcoholism
Chronic kidney disease
Decreased red blood cell pH |
Some genotypes |
| Altered haemoglobin |
|
|
Fetal haemoglobin
Haemoglobinopathies
Methaemoglobin |
| Assays |
Hyperbilirubinaemia
Carbamylated haemoglobin
Alcoholism
Aspirin (large doses)
Chronic opiate use
Hydroxyurea |
Hypertriglyceridaemia |
Haemoglobinopathies |
What if the HbA1c is unreliable?
It may become clear that the HbA1c is not reflecting average glycaemia because the average of blood glucose results (by BGM and laboratory) is very different from those expected from the HbA1c result: average BGL (mmol/L) = 1.59 x HbA1c% – 2.59, or more simply, = 2 HbA1c – 6.8
Clinical review might indicate some remediable medical condition (Table 2) and the laboratory may be able to use another assay method which will make the HbA1c reliable. If not, there are two alternatives to HbA1c testing to monitor overall glycaemia:
- self BGM before and after meals, checking that results are similar to those from laboratory testing of a blood sample taken at the time of self BGM
- testing other glycated proteins that reflect the average glycaemia during the lifetime of the proteins in the circulation (termed 'fructosamines', because after glycosylation glucose is converted to fructose). These proteins turn over much faster than red cells and reflect the overall blood glucose over the preceding 1–2 weeks. As for HbA1c, the relationship between fructosamine levels and average blood glucose is affected by changes in the lifespan of the proteins. A Medicare rebate is available for the test up to four times in 12 months,1 but check for local laboratory availability.
Future directions in HbA1c testing
There are two developments in diagnosis and reporting that may be implemented in Australia in 2012–2013:9
- the American Diabetes Association recommends HbA1c as well as venous plasma glucose levels to diagnose type 2 diabetes with diagnostic values of ≥6.5%
- internationally there is a move toward a new standard based on the chemistry of HbA1c (Table 3). If this change is adopted in Australia, both the old and new values of HbA1c will be reported initially.
There may also be a move toward more point-ofcare testing which is being used in some areas of Australia, particularly remote settings.10
The following case studies illustrate instances where HbA1c and self BGM results do not match.
Table 3. Comparison between HbA1c values under old and new reporting units11
| Current HbA1c (%) | New HbA1c in mmol/mol |
|---|
| 4.0 |
20 |
| 5.0 |
31 |
| 6.0 |
42 |
| 6.5 |
48 |
| 7.0 |
53 |
| 7.5 |
59 |
| 8.0 |
64 |
| 9.0 |
75 |
| 10.0 |
86 |
| 11.0 |
97 |
| 12.0 |
108 |
Case 1. Beverley
Reported BGM results: 4–7 mmol/L; HbA1c: 8.6%; estimated average blood glucose = 1.59 x 8.6 – 2.59 = 11.1 mmol/L.
What is happening?
Beverley was only testing the fasting blood glucose (generally the lowest over the 24 hour period). Tests at other times were much higher, explaining the HbA1c result.
Case 2. John
Reported BGM results: 8–13 mmol/L over the day; HbA1c: 6.2%; estimated average blood glucose = 1.59 x 6.2 – 2.59 = 7.3 mmol/L.
What is happening?
John had haemoglobinopathy, causing a spuriously low result in the laboratory's usual testing method. With a different assay system, the HbA1c was 8.4% with estimated average blood glucose of 10.8 mmol/L.
Conflict of interest: none declared.