The objective of the exploratory study reported in this article was to assess microbiological contamination in a sample of spirometers taken from South Australian general practices.
Methods
In 2009, we swabbed 16 spirometers from a convenience sample of metropolitan general practices in the Adelaide area of South Australia. Details of the spirometers (Table 1) and their use were obtained by questionnaire. The spirometers were swabbed in their 'ready to use' state in the practice. The sampling of the spirometers was done aseptically, ie. using sterile swabsticks that touched only the sampling area. Conditions in the laboratory were also aseptic.
Swabs from the turbine, mesh, mouthpiece tube or flow head were taken, placed into Amies transport medium and shipped to the Institute of Medical and Veterinary Science (Adelaide) within 12 hours after sampling. Swabs were inoculated onto a horse-blood agar plate, a chocolate agar plate (ie. a blood agar plate in which the blood has been 'chocolatised', which releases more nutrients for organisms to utilise) and a cysteine lactose electrolyte deficient (CLED) agar plate (which supports the growth of both Gram positive and Gram negative isolates). The blood and chocolate plates were incubated at 35°C in an atmosphere containing 5% CO2 while the CLED plate was incubated aerobically, also at 35°C. After 24 and 48 hours incubation, colony types present were identified by appearance on the inoculated medium and by Gram stain of the growing colonies, followed by testing the isolates against a battery of biochemical tests, including sugar utilisation tests.
We consulted the 2007 revised National Statement on Ethical Conduct in Human Research,6 and because no human subjects were involved in the study, did not consider that ethics approval was required.
Results
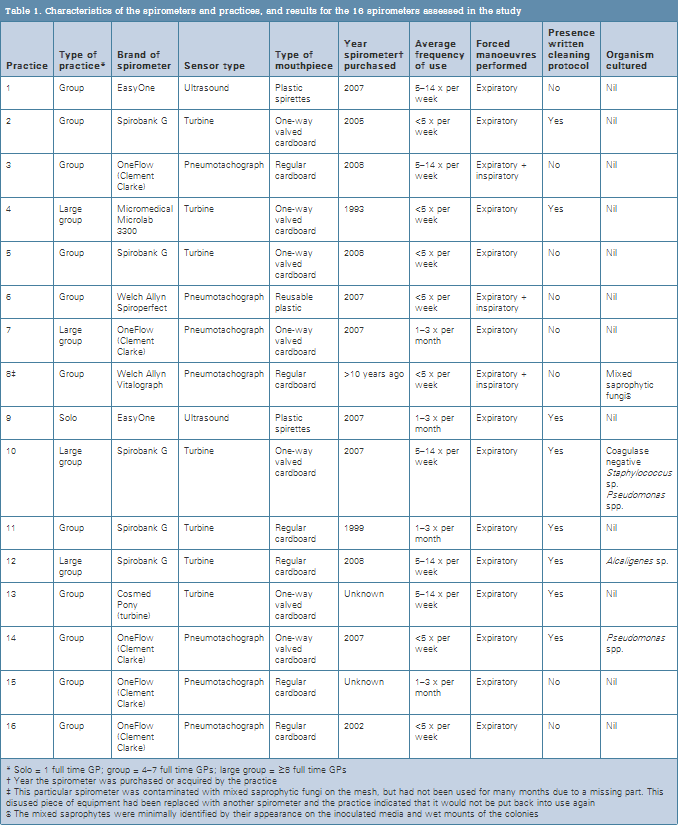
Eight of the practices (50%) reported having a spirometer cleaning protocol in place. Three practices used spirometers that had disposable flow heads. None of the other practices used bacterial filters, with seven using one way valved disposable cardboard mouthpieces. Reported frequency of spirometer cleaning varied between once per week (in one practice) to 4–6 times per year (in two practices).
Three spirometers carried potentially pathogenic micro-organisms (Table 1): two Pseudomonas spp., one coagulase negative Staphylococcus sp. and one Alcaligenes sp. All three spirometers had been in use for less than 3 years, all three practices had written cleaning protocols in place and all stated that they were using a recommended detergent for cleaning. Two practices used one-way disposable cardboard mouthpieces and one used disposable (non-one-way) cardboard mouthpieces. None of the three practices with contaminated spirometers performed inspiratory manoeuvres.
Discussion
We found potentially relevant microbiological contamination in 3 out of 16 general practice spirometers. This occurred despite the fact that these practices had relatively new spirometers and had written cleaning protocols in place. However, the practices' cleaning protocols did not match the manufacturers' recommendations regarding the frequency of disinfection: according to the manuals disinfection would need to be carried out 'prior to every use' (for the Spirobank G spirometer)7 or 'weekly' (for the OneFlow spirometer when used with disposable one-way cardboard mouthpieces),8 respectively. All three practices reported a lower cleaning frequency than recommended. On the other hand, none of the three practices performed inspiratory manoeuvres, which would probably have posed more of a risk.
The potential hazard of spirometers as reservoirs of micro-organisms stresses the need for stricter attention to hygiene measures for spirometers in general practices, since we don't know the likelihood of pathogens being breathed in by subsequent patients. Limitations of this exploratory study were: the small sample size; no negatives controls (eg. swabbing with sterile water) were collected; no viral sampling was performed; there was an (inevitable) delay of a maximum of 12 hours between sampling and incubation; and the microbes found on the spirometers were not characterised in sufficient detail to determine whether or not they were human pathogens or nonpathogenic environmental species.
Nonetheless, until further research clarifies this risk, we strongly recommend that general practices implement and adhere to a strict protocol for spirometer cleaning following the manufacturers' instructions and use appropriate barrier filters in order to prevent equipment contamination with and cross-transmission of micro-organisms. Alternatively, practices can choose to switch to a spirometer that uses disposable flow heads.
Conflict of interest: none declared.