Paediatric-onset inflammatory bowel disease is on the rise
International trends have confirmed a global rise in the incidence of paediatric-onset inflammatory bowel disease (IBD); in particular, there has been a steep rise in the incidence of Crohn’s disease.1 Two Australian observational studies have confirmed similar trends, with a dramatic rise in paediatric-onset IBD over the past two decades.2,3 Approximately 10–20% of IBD cases are diagnosed during childhood, with peak age of onset at 15–29 years. The reasons behind the increase in IBD are poorly understood and cannot be completely explained by rising health awareness or better access to health services.
Diagnosis of IBD in adolescents
The most common presenting symptoms in paediatric Crohn’s disease are abdominal pain, diarrhoea and weight loss; however, one in four children has non-specific symptoms, including lethargy, anorexia, delay in linear growth and pubertal failure.4 On the other hand, bloody diarrhoea is the most consistent presentation of ulcerative colitis. Extra-intestinal manifestations of IBD, including arthralgia, aphthous ulcers and erythema nodosum, are seen in one-quarter of children, and are associated with increasing disease severity.5
The diagnosis of IBD is based on a combination of clinical presentation and examination, and abnormal laboratory screening tests. While the presence of anaemia, elevated inflammatory markers, including erythrocyte sedimentation rate and C-reactive protein (CRP), are helpful in the right clinical context, a normal biochemistry can be seen in 21–50% of children with IBD.6 Less invasive stool tests, such as faecal calprotectin, are extremely useful screening tools for IBD and avoid unnecessary endoscopy; however, it is not covered under the Australian Medicare Benefits Schedule.7 All children with suspicion of IBD should undergo a complete endoscopic evaluation, including ileocolonoscopy and upper gastrointestinal endoscopy with multiple biopsies. Small bowel imaging should be performed at diagnosis in all adolescents with IBD, except in those with definitive ulcerative colitis, for accurate classification of disease extent and to rule out complicating Crohn’s disease (strictures and fistulising disease). Persistently abnormal liver biochemistry in children should be further investigated for the possibility of immune-mediated liver disease (IMLD), including autoimmune hepatitis, autoimmune sclerosing cholangitis (overlap of autoimmune hepatitis and sclerosing cholangitis) and primary sclerosing cholangitis (PSC). IMLD can present or precede IBD in children, and PSC is more commonly associated with paediatric ulcerative colitis and Crohn’s disease.
IBD is a spectrum
Traditionally, IBD was classified as Crohn’s disease, ulcerative colitis or a true indeterminate colitis type with overlapping features of Crohn’s disease and ulcerative colitis, also known as IBD-unclassified (IBD-U). However, it is increasingly helpful to describe IBD as a heterogeneous group of chronic intestinal inflammatory conditions in children, as recent genetic research has cast new light on these old classifications. Not only is colonic Crohn’s disease more genetically similar to ulcerative colitis than to ileal Crohn’s disease, but studies in young children confirm the existence of specific monogenetic/immunological defects in up to 20% of IBD starting in the first two years of life.8
IBD in children has extensive distribution and aggressive behaviour despite use of more medications
Typically, Crohn’s disease is characterised by a transmural inflammation with skip lesions involving anything from mouth to anus, whereas ulcerative colitis is continuous colonic mucosal (more superficial) involvement from rectum to caecum. Cohort studies confirm that, compared with adult-onset IBD, paediatric-onset IBD (Crohn’s disease and ulcerative colitis) is more severe in nature, with panenteric involvement, rapid progression and increased disease activity, despite use of more immunosuppression (Table 1).9–11 Not only is the disease phenotype severe, there are additional issues of growth failure, delayed puberty, poor bone density and psychosocial impact at a vulnerable period of life.
Linear growth delay preceding the onset of gastrointestinal symptoms and 5–8 cm loss of final adult height has been reported, despite optimal treatments.12 The expanding treatment paradigms in the management of Crohn’s disease over the past two decades had not changed the prevalence of growth failure until the most recent reports in centres using more intensive treatment approaches.13
Table 1. Key difference between paediatric-onset and adult-onset inflammatory bowel disease
|
Crohn’s disease8,9
|
Paediatric-onset
|
Adult-onset
|
|---|
|
Ileocolonic and upper gastrointestinal involvement (panenteric)8
|
43%
|
3%
|
|
Cumulative disease activity over 10 years9
|
37%
|
31%
|
|
Use of immunomodulators over 10 years9
|
54%
|
45%
|
|
Risk of intestinal resection at 30 years9
|
48%
|
14%
|
|
Risk of permanent stoma9
|
12%
|
1%
|
|
Ulcerative colitis8,10
|
Paediatric-onset
|
Adult-onset
|
|---|
|
Pancolitis
|
70–82%
|
19–48%
|
|
Mild disease course
|
35%
|
72%
|
|
Corticosteroid-dependence
|
45%
|
8%
|
|
10-year risk of colectomy
|
40.9%
|
19.9%
|
Treatment targets
Control of symptoms or healing the mucosa?
To choose the best therapeutic targets, it is important to conceptualise a stepwise progression of paediatric-onset IBD from a very early, pre-symptomatic stage that will last for several years, to full-blown symptomatic disease before diagnosis and progressive intestinal damage and growth failure (Figure 1).
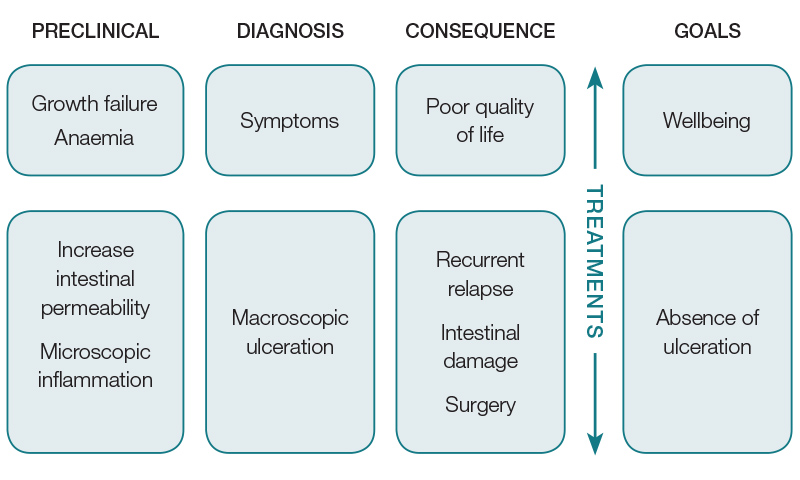
Figure 1. Schematic presentation of inflammatory bowel disease progression
Observational studies indicate that 70–80% of patients who have Crohn’s disease with ulceration on endoscopy report no symptoms; hence, controlling symptoms alone is inadequate as progressive intestinal damage (with future worsening of symptoms) will not be prevented.14
On the other hand, many patients with Crohn’s disease and mucosal healing will report IBD-like symptoms of lethargy, pain and diarrhoea, for which escalation of disease treatments will increase risks without improving quality of life by missing more appropriate treatments.
Mucosal healing, defined as the absence of ulceration in Crohn’s disease and ulcerative colitis, has emerged as an important end-point that, when achieved early, leads to less frequent relapses, fewer hospitalisations, reduced surgical resections and lower risk of Crohn’s disease fistulising disease and, hence, less intestinal damage.15–17 It also leads to higher quality-of-life scores and reporting of ‘feeling normal’ even in so-called asymptomatic patients. Prospective paediatric studies are few but suggest that early repeat endoscopy to confirm healing is feasible, and better long-term outcomes in Crohn’s disease at one, two and three years can be achieved in those achieving early healing of the intestinal mucosa.18,19
While the best long-term outcomes are only possible by achieving and maintaining mucosal healing, symptom control is also vital for improving immediate quality of life. Thus, the goal for treatments should include the combination of controlling symptoms and mucosal healing, also referred to as ‘deep remission’. The US Federal Drug Administration (FDA) now mandates that deep remission should be used as the treatment target for all new IBD-related drug trials, to prove therapeutic efficacy.20
Treatment options for children with IBD
The choice of treatment is influenced by disease behaviour (inflammatory or complicated with stricture or fistula), severity, growth failure and presence of extra-intestinal manifestations. Treatment efficacy should include improvements in symptoms and growth, reduction in inflammatory biomarkers such as CRP, faecal calprotectin and, most importantly, healing of the mucosa.
Therapies for IBD are rapidly evolving and there are many newer agents on the horizon, especially ‘biologic agents’ (ie antibodies to specific proteins involved in the inflammatory process). For the purpose of this article, we have outlined the current approach, including medications currently licenced and available for use in Australia (Table 2).
Under our current funding scheme, anti−tumour necrosis factor (TNF) agents cannot be accessed for paediatric IBD without failing an adequate trial of conventional therapies (exclusive enteral nutrition, corticosteroids and/or immunomodulators in Crohn’s disease; and oral aminosalicylic acid/corticosteroids and immunomodulators in ulcerative colitis) for three or more months. The only exceptions to this rule are acute severe colitis (three doses of rescue biologics to avoid colectomy), complex perianal fistula and significant intolerance to the above-mentioned therapies. Recent evidence from a North American study suggests superior clinical and growth outcomes can be achieved in paediatric Crohn’s disease for those commencing early anti-TNF biologic therapy (less than three months after diagnosis).13 These observations need further validation as not all children will require anti-TNF agents within three months, and perhaps a risk-based individualised approach is required.
Table 2. Treatment of inflammatory bowel disease
|
Crohn’s disease
|
|---|
|
Induction therapy:
- EEN therapy (exclusive oral polymeric diet for eight weeks)
- First-line therapy
- Greater mucosal healing rates and nutritional benefits
- Corticosteroids (prednisolone 1 mg/kg, maximum 60 mg/daily, taper over six to eight weeks)
- Similar efficacy in controlling symptoms but poor mucosal healing
Maintenance therapy (slow-acting, benefit observed only after 10–12 weeks):
- Oral (AZA) 2–2.5 mg/kg or subcutaneous (MTX) 10–20 mg/m2
Biologics (anti-TNF agents – infliximab and adalimumab):
- Faster acting, higher mucosal healing rates
- Used for induction and maintenance therapy
- Adalimumab: induction dose 160 mg at week 0 and 80 mg at week 2; maintenance therapy with 40 mg every other week (>40 kg)
- Infliximab: induction dose 5 mg/kg at weeks 0, 2 and 6 weeks; maintenance therapy every 8 weeks
- Indications:
- First-line therapy for externally draining complex perianal fistula
- Failure to respond to or tolerate above therapies in moderate‑to‑severe active Crohn’s disease
|
|
Ulcerative colitis
|
|---|
|
Acute severe colitis (hospitalised patient):
- Intravenous methylprednisolone 1 mg/kg/day (maximum 60 mg)
- No response day 3–5 to prednisolone
- Infliximab 5 mg/kg/dose (PBS-subsidised; two further infliximab doses)
- Poor response to first infliximab dose
- Consider early second dose (<1 week) or consider calcineurin inhibitors cyclosporine A/tacrolimus, especially in those losing large amounts of protein-bound drug (eg infliximab) into stool
- Subtotal colectomy
- Patients with severe active disease who do not respond to corticosteroid/biologics
Mild-to-moderate ulcerative colitis:
- Oral 5-ASA:
- First-line therapy used for induction and maintenance of remission
- Topical (rectal) 5-ASA therapy if mild-to-moderate proctitis alone
Moderate-to-severe ulcerative colitis (non-hospitalised):
- Oral prednisolone 1 mg/kg/day, maximum 60 mg, for inducing remission but not for maintaining remission in those with severe disease or failure to respond to 5-ASA
- Consider AZA or 5-ASA as a maintenance therapy in those treated with prednisolone
Moderate-to-severe ulcerative colitis (failed steroids + AZA and oral 5-ASA therapy):
- Infliximab 5 mg/kg/dose at 0, 2, 6 and 8 weekly maintenance
- Adalimumab 160 mg induction, 80 mg week 2 and 40 mg weekly (>40 kg)
|
|
ASA, aminosalicylic acid; AZA, azathioprine; EEN, exclusive enteral nutrition; MTX, methotrexate; PBS, Pharmaceutical Benefits Scheme; TNF, tumour necrosis factor
|
Can we avoid repeat endoscopy and use surrogate biomarkers of mucosal healing as treatment targets?
In ulcerative colitis, a symptom severity score, the Paediatric Ulcerative Colitis Activity Index (PUCAI), has been validated against endoscopic disease activity, and is a useful objective score to monitor disease activity and response to therapy. However, in Crohn’s disease, recognition that abdominal pain and day-to-day symptoms can be easily influenced by emotional wellbeing and do not correlate with inflammation has decreased confidence in the PCDAI and increased interest in surrogate biomarkers of inflammation. Biomarkers such as serum CRP and faecal calprotectin are increasingly used in day-to-day practice. Faecal calprotectin is a neutrophilic protein that migrates from the site of intestinal inflammation to be excreted, unaffected by the intestinal microbiota, in stools. Higher concentrations (>100 µg/g of stool) indicate greater inflammation in the bowel and increased likelihood of disease relapse or of coexisting or precipitating intestinal infections, contributing to mucosal inflammation.
Prospective endoscopic studies report serum CRP and faecal calprotectin to be more reliable than clinical symptoms in predicting Crohn’s disease mucosal inflammation.21 However, CRP is elevated in only half of the patients who have Crohn’s disease with endoscopic-proven inflammation, and not all patients with raised faecal calprotectin have evidence of IBD ulceration at ileocolonoscopy. Unlike in Crohn’s disease, CRP rise in ulcerative colitis is mild or commonly absent, presumably reflecting the superficial, mucosal ulceration that characterises most ulcerative colitis. Faecal calprotectin is more reliable in monitoring ulcerative colitis disease activity, compared with Crohn’s disease.22 Thus, PUCAI and faecal calprotectin are useful tools for monitoring ulcerative colitis disease activity. However, although useful, CRP and faecal calprotectin are not sufficiently accurate to serve as primary end-points for the treatment of Crohn’s disease. This leaves endoscopy as the gold standard measure of Crohn’s disease activity at the present time. This is increasingly used to establish therapeutic efficacy in day-to-day clinical practice.
Mucosal examination on endoscopy alone may be sufficient for documenting mucosal disease activity in ulcerative colitis; however, it does not provide complete information regarding transmural inflammation in Crohn’s disease or extension of disease to the proximal small bowel. This is where non-invasive, cross-section small bowel imaging techniques are attractive. Computed tomography scanning is now giving way to magnetic resonance enterography (MRE), which gives excellent pictures, avoids radiation exposure, requires no general anaesthetic in children six years of age and older, and provides information regarding transmural intestinal disease activity in Crohn’s disease. The overall sensitivity of MRE for the detection of disease activity is 80% (95% confidence interval [CI]: 77%, 83%) and specificity is 89% (95% CI: 93%, 96%).23 Emerging data suggest that better outcomes in Crohn’s disease are associated with transmural healing (MRE-confirmed healing), but <15% of patients are able to achieve this end-point.24 However, access to MRE is limited by its popularity in all medical specialities and the need for special facilities to house complex and expensive hardware. Intestinal ultrasonography is now gaining popularity in IBD specialist centres for more immediate, bedside, cross-sectional imaging to monitor patients.
Unique issues in adolescents with IBD
There are several challenges in the management of adolescents with IBD, a chronic lifelong intestinal disease at a vulnerable period of psychosocial development. Unpleasant symptoms related to disease and medications, peer pressure and parental preconceptions of risks often dictate treatment decisions and medication adherence.
Poor medication adherence can be minimised by clearly communicating the benefits of maintenance therapy, even in the absence of symptoms, at follow-up appointments by healthcare providers. Shared-care management plans involving general practitioners (GPs), with clear documentation of anticipated side effects and phone support, can improve surveillance of toxicity (Table 3), rare infections and malignancy.
It is important to communicate the risk of therapies to adolescents as it is a very emotional and time-consuming subject, which is complicated by misinterpretation of medical data. When explaining to adolescents the risk of lymphoma or malignancy on exposure to thiopurines or anti-TNF agents, a visual frequency display is more intuitive than describing relative risks (eg a twofold increase in risk of malignancy on thiopurines seems more concerning than an increase from two in 10,000 to four in 10,000).
A multidisciplinary, holistic model of care, including psychologists, dietitians, IBD-focused clinical nurses and physicians (eg GPs, gastroenterologist, surgeons) is critical for achieving the best outcomes. Discussions to minimise risk-taking behaviours, including smoking, alcohol and sex, are key elements for a successful transition from family and growth-centred paediatric care to an adult facility with greater emphasis on autonomy, employment and cancer surveillance.
Table 3. Toxicity monitoring in adolescents with IBD
|
Drug
|
Side effects
|
|---|
|
Aminosalicylic acid compounds
|
Headache, nausea, epigastric discomfort, diarrhoea
Serious rare – Steven–Johnson syndrome, pancreatitis, agranulocytosis, interstial nephritis
|
|
Corticosteroids
|
Acne, facial swelling, weight gain, increase appetite, sleep and mood disturbance, dyspepsia, glucose intolerance
Long term – Cataracts, osteoporosis, osteonecrosis of hip, increase susceptibility to infections
|
|
Azathioprine
|
Nausea, vomiting, diarrhoea, muscle aches
Monitor liver function and full blood counts every week for the first month, every two weeks for the next two months and every three months thereafter
Infections, including lymphoma and pancreatitis, have been rarely reported
Monitoring – elevated 6-thioguanine (>450 pmol/8 x 108 RBCs, myelotoxicity) and methyl mercaptopurine (>5700 >450 pmol/8 x 108 RBCs, hepatotoxicity)
|
|
Methotrexate
|
Nausea, hair loss, headache, dizziness, drowsiness, mouth sores
Conception on methotrexate may cause birth defects
Hepatotoxicity and pneumonitis
|
|
Anti-TNF
|
Injection site reactions, including redness, rash, swelling, itching, pain, bruising
Infusion reactions – mild-to-serious anaphylaxis and delayed reactions such as myalgia, fever
Reports of serious infections associated with anti-TNF agents, including tuberculosis and other bacterial infections, and viral and fungal infections, that have spread throughout the body
Reports of increased risk of cancer, including lymphoma
Recent paediatric data suggest no increased risk of malignancy or lymphoproliferative disease on anti-TNF alone in children with IBD27
|
|
IBD, inflammatory bowel disease; RBCs, red blood cells; TNF, tumour necrosis factor
|
Vaccination in children with IBD
Children with IBD on immunomodulating drugs (eg corticosteroids, azathioprine, methotrexate or biologics) remain at high risk of opportunistic infections; therefore, it is vital to minimise this risk by optimising vaccination (Table 4). Most children in Australia will have been vaccinated prior to the time of diagnosis; however, a good vaccination and travel history at diagnosis is vital. All children newly diagnosed with IBD should have vaccination titres tested for hepatitis B and C, varicella serology, and quantiferon tuberculosis.
If immunity cannot be established, the child should have their vaccination schedule updated as soon as possible while on non-immunosuppressive treatments, such as exclusive enteral nutrition in Crohn’s disease and 5-aminosalicylic acid in ulcerative colitis. Once the child has started immunosuppressive agents and/or biologics, the use of live vaccinations (eg measles, mumps and rubella [MMR], varicella, yellow fever) is contraindicated and the response to non-live vaccinations, such as hepatitis B, is attenuated.
For those contemplating commencing immunosuppression, there should be a gap of at least four to eight weeks after the live vaccination.25 For those on immunosuppression considering a treatment break, medication should be ceased at least 12 weeks before, and not recommenced for at least four weeks after the live vaccinations. Household members can receive MMR and varicella live vaccine, but if vaccine‑related rash develops in contacts, there is risk of transmission to patients with IBD who have no immunity to varicella.25 Clear communication between treating teams and GPs is essential for optimal vaccination schedules and minimising risk of opportunistic infections.
Table 4. Vaccination in adolescents with inflammatory bowel disease25
|
Vaccination
|
Note
|
|---|
|
Hepatitis B (non-live)
|
If non-immune, give three doses
|
|
Diphtheria and tetanus (non‑live)
|
Vaccinate if not given in the past 10 years
|
|
Influenza (non-live)
|
Consider annual vaccination
|
|
Pneumococcal (non-live)
|
Consider vaccination every five years
|
|
Human papillomavirus (non-live)
|
Should be offered
|
|
Meningococcal (non-live)
|
Should be offered
|
|
Live vaccines – varicella, or measles, mumps, rubella
|
If not immune or unvaccinated
Can be given, but a minimum eight-week interval is suggested before commencing immunomodulators or biologics
|
Role of patients support group and well‑structured transition process
The psychosocial development process in teenagers with IBD is delayed, compared with peers, and this could have a negative impact on their adjustment to independent life.26 The implications of psychosocial immaturity are loss of self-esteem, high dependence on caretakers and lack of future employment.26 A well-structured transition process from paediatric to adult gastroenterologist involving GPs, psychologist and family support is crucial for smooth adjustment to adult life.
Crohn’s & Colitis Australia (CCA) and teenage camps are pivotal in extending support, comfort and education to teenagers with IBD and their family. Participation in summer camps organised by CCA is associated with improvement in disease-specific knowledge, and may lead to better psychosocial adjustment to unresolved grief of IBD.
Conclusion
Despite expanding treatments paradigms, it is clear that long-term outcomes are dependent on quality of management and reaching mucosal healing early in the course of the IBD. Growth failure and pubertal delay are common extra-intestinal health symptoms in children with IBD, and their recognition is vital for early intervention. Shared care with GPs, written management plans, supportive education tools for GPs, phone access for patients and GPs to specialist IBD nurses and teams, and deeper healing, are currently the best strategies to improve the care of children with IBD.
Authors
Zubin Grover MD, FRACP, Consultant Gastroenterologist and Staff Specialist, Princess Margaret Hospital for Children, Subiaco, WA. zubingrover@gmail.com
Angela De Nardi RN, Clinical Nurse Specialist for Inflammatory Bowel Disease, Princess Margaret Hospital for Children, Subiaco, WA
Peter J Lewindon FRACP, Associate Professor, School of Paediatrics and Child Health and Senior Consultant Paediatric Gastroenterologist, Lady Cilento Children’s Hospital, Brisbane, Qld
Competing interests: PL serves as a scientific advisory board member for AbbVie and Janseen, and received lecture fees from Janseen and AbbVie.
Provenance and peer review: Commissioned, externally peer reviewed.
Acknowledgments
ZG received a Queensland Children and ANZ Trustees PhD scholarship.