Hyperlipidaemia is one of the most common problems managed by general practitioners (GPs) and the majority of lipid-lowering drugs are prescribed by GPs.1 Statins have been shown to effectively reduce the risk for cardiovascular events and all‑cause mortality in men and women, and are generally considered safe.2 Nevertheless, adherence to statin therapy is suboptimal.3 Many barriers, such as concerns about adverse events, inadequate information, uncertainty about drug efficacy and the media, potentially influence adherence to treatment.4,5 For example, it was estimated that 60,000 consumers discontinued their prescribed anti-cholesterol medication as a direct result of the Catalyst program titled ‘Heart of the matter’.5 The program questioned the link between high cholesterol levels and cardiovascular disease (CVD), and suggested that the benefits of statins had been overstated and harms downplayed.
Despite statins being so commonly used, little is known about the ‘real-world’ information needs of consumers using statins. The majority of studies invite statin users to reflect on their concerns,4,6 whereas to our knowledge, no study to date has explored spontaneous uninvited questions by consumers.
The aim of this study was to explore consumers’ questions about statins to a national medicines call centre in Australia. Our findings can assist GPs to provide more consumer-relevant medicines information that facilitates shared decision-making.
Methods
Service model and database
We used data from the Australian NPS MedicineWise (formerly the National Prescribing Service Medicines Line), collected between September 2002 and June 2010. This call centre, operated by clinical pharmacists, was available nationwide to consumers with medicines‑related questions.
Calls were documented using a standardised form. These included demographic characteristics of caller and patient, relationship of caller to patient, and motivation for calling the service. For each call, up to three generic medicines related to the caller’s question were recorded. The narrative of the main question was recorded, but these were only electronically recorded in the database from 2007 until 2010.
Medicines were classified according to the Anatomical Therapeutic Chemical (ATC) classification system.7 For this paper, we included all calls that had at least one medication in the ATC level 4 group C10AA (ie HMG-CoA reductase inhibitors) further referred to as statin‑related calls).
This study was approved by Mater Health Services Brisbane’s Human Research Ethics Committee (LNR submission 2012–68).
Analyses
Call characteristics and medicines most commonly enquired about in statin-related calls were compared with the rest of the calls using Student’s t-test or chi square test. Throughout all analyses, a P value <0.05 was considered statistically significant. Statistical analyses were performed using SPSS version 22.8
Two authors (LD, SK) independently identified all concerns of electronically available question narratives from statin‑related calls. These were used to develop a coding scheme and discordances were resolved through discussion. Subsequently, concerns were collapsed into overarching categories on the basis of frequency of occurrence. Separate concerns that accounted for <1% of the statin-related calls were categorised in the subgroup ‘other’ for each overarching category (eg the overarching category ‘interaction’ has a subgroup ‘other interactions’). Statin-related calls in which the question was clearly not focused on the statin were excluded. In these calls, although callers mentioned a statin, their concern was not related to the statin.
Subsequently, we evaluated whether the concerns emerging from the questions were also included in the consumer medicine information (CMI), which was available for the statins most commonly enquired about (ie atorvastatin and simvastatin) at the time of data collection. We also formulated answers to these questions using the Australian Medicines Handbook as our main resource.9
Table 1. Demographics of statin-related calls and drugs and drug classes most enquired about1
| Statin-related calls n = 3,555
| Rest of calls n = 119,662 | P value |
|---|
|
|
n
|
%
|
|
n
|
%
|
|
|
Gender of caller
|
|
|
|
|
|
|
<0.001
|
|
Male
|
|
1,036
|
29%
|
|
27,356
|
23%
|
|
|
Female
|
|
2,518
|
71%
|
|
92,115
|
77%
|
|
|
Missing
|
|
1
|
0%
|
|
191
|
0.2%
|
|
|
Relationship of caller
|
|
|
|
|
|
|
<0.001
|
|
Self
|
|
2,875
|
81%
|
|
85,135
|
71%
|
|
|
Partner
|
|
370
|
10%
|
|
6,571
|
5%
|
|
|
Other
|
|
306
|
9%
|
|
27,589
|
23%
|
|
|
Missing
|
|
4
|
0%
|
|
367
|
0%
|
|
|
Location (ARIA code)
|
|
|
|
|
|
|
0.74
|
|
Major cities
|
|
2,609
|
73%
|
|
88,596
|
74%
|
|
|
Inner regional Australia
|
|
593
|
17%
|
|
19,548
|
16%
|
|
|
Outer regional Australia
|
|
266
|
8%
|
|
8,372
|
7%
|
|
|
Remote Australia
|
|
36
|
1%
|
|
1,313
|
1%
|
|
|
Very remote Australia
|
|
13
|
0.4%
|
|
393
|
0.3%
|
|
|
Missing
|
|
38
|
1%
|
|
1,440
|
1%
|
|
|
Motivation to call
|
|
|
|
|
|
|
<0.001
|
|
Inadequate Information
|
|
1,392
|
39%
|
|
56,069
|
47%
|
|
|
Worrying symptom
|
|
907
|
26%
|
|
20,996
|
18%
|
|
|
Second opinion
|
|
818
|
23%
|
|
28,117
|
24%
|
|
|
Conflicting Information
|
|
174
|
5%
|
|
6,697
|
6%
|
|
|
Other
|
|
264
|
7%
|
|
7,783
|
7%
|
|
|
Missing
|
|
5
|
0%
|
|
307
|
0%
|
|
|
Top five most common drugs classes (ATC level 4)*
|
|
|
|
|
|
|
<0.001 |
|
Platelet aggregation inhibitors excluding heparin (B01AC) 351
|
|
10%
|
2,600
|
|
2%
|
|
|
ACE inhibitors, plain (C09AA)
|
|
350
|
10%
|
|
2,724
|
2%
|
|
|
Angiotensin II antagonists, plain (C09CA)
|
|
257
|
7%
|
|
2,889
|
2%
|
|
|
Beta-blocking agents, selective (C07AB)
|
|
236
|
7%
|
|
2,040
|
2%
|
|
|
Proton pump inhibitors (A02BC)
|
|
173
|
5%
|
|
3,217
|
3%
|
|
|
Top five most common drugs (ATC level 5)*
|
|
|
|
|
|
|
<0.001 |
|
Aspirin (B01AC06)
|
|
247
|
7%
|
|
2,018
|
2%
|
|
|
Irbesartan (C09CA04)
|
|
151
|
4%
|
|
1,495
|
1%
|
|
|
Atenolol (C07AB03)
|
|
150
|
4%
|
|
1,235
|
1%
|
|
|
Perindopril (C09AA04)
|
|
147
|
4%
|
|
1,305
|
1%
|
|
|
Ramipril (C09AA05)
|
|
133
|
4%
|
|
775
|
1%
|
|
|
*Each call recorded up to three drugs: top five most common, other drugs, and drug classes enquired about in statin-related calls
ACE, angiotensin converting enzyme; ARIA, Accessibility/Remoteness Index of Australia; ATC, Anatomical Therapeutic Chemical classification
|
Results
Between September 2002 and June 2010, a total of 123,217 calls were recorded, of which 3555 (3%) were related to statins. The majority of statin-related calls concerned atorvastatin (53%), followed by simvastatin (28%) and rosuvastatin (13%).
The majority of calls were from female patients (71%). However, compared with the rest of the calls, statin-related callers were more often male (P <0.001), and more often called for themselves or their partner (P <0.001). The mean age of callers with statin-related questions was 62 years (standard deviation = 13.55). The main motivation for calling was that there was inadequate information (39%). Some people called after obtaining information from a doctor (6%) or a pharmacist (2%). However, for the majority of callers, this information was missing (83%). Besides statins, callers also mentioned other medications; those most commonly enquired about are summarised in Table 1.
Of the 3555 statin-related calls, the question narratives were electronically available from 2007 to 2010 (ie available for 50% of the calls; n = 1778). Of these, 292 calls were excluded because the question was not focused on the statin, leaving 1486 for further analysis (Figure 1). The most common concerns related to side effects of medicines and interactions, which accounted for 36% and 28% respectively. Callers’ concerns about side effects primarily related to musculoskeletal (27%), gastrointestinal (12%) and skin problems (5%). Callers often desired individualised information. Typical questions included:
Could simvastatin be causing my muscle pains? Could simvastatin have caused my rash?
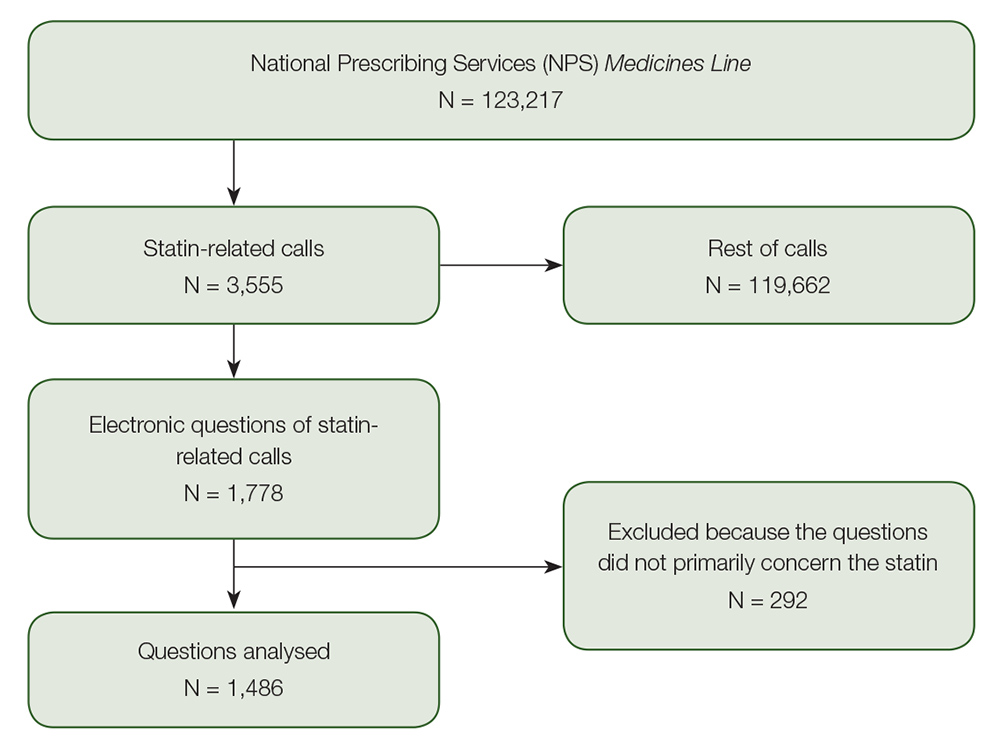
Figure 1. Flowchart for inclusion of statin-related calls in analyses
Common concerns about interactions related to statins possibly interacting with other medicines (49%), complementary and alternative medicines (CAMs; 39%), and grapefruit (6%). Typical questions included:
Can I take colchicine with atorvastatin and candesartan? Can he take Echinacea with atorvastatin?
Other common concerns related to general information needs (14%), and included questions about the necessity to take statins and stopping medication. Questions about the different treatment options (12%) included, for example, questions about changing between statins, such as:
Can my partner swap between atorvastatin and simvastatin?
Further details are summarised in Table 2.
The topics of most concern were those mentioned in the CMI (Table 2). For example, except for weight gain and memory problems, most concerns relating to side effects and interactions were listed in the CMI. However, therapeutic strategies to prevent or manage these concerns were less often discussed in the CMIs. For most side effects, it was generally stated that these can occur, with advice to ‘tell your doctor immediately or go to the casualty department of your nearest hospital’. Similarly, for CAMs, it was advised to ‘tell your doctor if you are taking other medicines, which include vitamins and supplements that are available from your pharmacy, supermarket or health food shop’.
For overdose, CMIs describe what to do when too much is taken; however, the dosage that is considered ‘too much’ was not defined. Similarly, for interactions, such as interactions with grapefruit, some CMIs merely state that large quantities increase the risk for side effects without specifying a safe dose.
Discussion
The main motivation to call was inadequate information and almost two‑thirds (64%) of statin-related calls focused on aspects of safety, including possible side effects or interactions with other prescription medicines or CAMs. Other common concerns related to general information needs, the possibility of stopping the medication, and changing between statins.
Despite statins being commonly used and generally considered safe, consumers have many concerns about their use, which contributes to statin hesitancy. Approximately 50% of consumers discontinue statins within the first year of starting treatment, and this number increases with subsequent years of treatment.10 The most common reasons for discontinuation of statins are consumer concerns about side effects, costs, uncertainty of benefits, and concerns about interactions.4,11 In addition, gaps in consumers’ education and unmet information needs predicted statin adherence.12 These reasons for non‑adherence align with the most common concerns identified in our study.
Non-adherence can have serious implications. A systematic literature review and meta-analysis estimated that approximately 9% of all CVD events could be attributed to poor adherence to cardiovascular medication alone.13 Hence, it is important that GPs – who are the main statin prescribers – understand and address consumers’ concerns adequately and in a timely manner to improve adherence.
The majority of concerns relating to side effects are listed in the CMI. For most side effects (eg skin rash, dizziness), the CMI generally recommends to ‘tell your doctor immediately or go to the casualty department of your nearest hospital’. The large number of calls on this issue – despite the topic being mentioned in the CMI – illustrates that consumers are insecure about symptoms that could be related to their medication. The advice in the CMI to ‘tell your doctor immediately’ could be compounding this insecurity. As patients with CVDs are commonly prescribed multiple medicines, and each CMI focuses on an individual medicine, it is not surprising that they seek information beyond statin medication leaflets. This provides an opportunity for GPs to address the consumer concerns identified in this study and personalise their statin counselling.
While the majority of interaction concerns were mentioned in the CMIs, direct applicability of the information differed across different products. For the consumption of grapefruit, some CMIs indicate that one glass of grapefruit juice a day is probably safe; however, others merely state that large quantities increase the risk for side effects, without specifying a safe dose. More specific and consistent consumer-level information in CMIs in this respect may alleviate concerns.
We also found that almost 40% of concerns about interactions with statins were about potential interactions with CAMs. CMIs generally refer consumers back to the doctor, with the advice to ‘tell your doctor if you are taking other medicines, which includes vitamins and supplements that are available from your pharmacy, supermarket or health food shop’. However, an Australian consumer survey found that only half of those using CAMs mentioned this to a doctor.14 Reasons cited for non-disclosure included a belief that CAMs are ‘natural’ and ‘safer’ than conventional medicines; discomfort in raising the topic; fear of the GP’s response; or simply because their clinician failed to ask.14,15 GPs often lack the knowledge or confidence to advise patients about the risks of taking CAMs.16,17 It may also explain why consumers took it upon themselves to seek help about the potential interactions between statins and CAMs. Therefore, it will become increasingly important that GPs become familiar with common and clinically significant CAM interactions, or at least know where to look up the information.18
We also found that consumers questioned the need to take a statin or wondered if they were likely to experience any benefits from taking statins. Tailored information by GPs, cardiologists and pharmacists could allay these concerns.
This study has important strengths. Questions were collected nationwide over eight consecutive years and captured real consumers’ needs for medicines information. Medicines Line was designed as a healthcare service, not as a research project, ensuring that we have ‘real-world’ data, where callers are worried enough to seek help (ie pick up the phone and call) and address their concern.
Using observational data routinely collected as part of a health service without specific a priori research goals for research is increasingly being recognised as a valid translational research approach, with international guidelines now published for the conduct of such research.19 This approach is particularly suited to medicines call centre studies.20 The downside of this approach is that callers to the service might not represent all patients who use statins and, instead, callers are more likely to be health‑conscious medicine users.21 In particular, women are overrepresented in the data, and they are more likely to proactively seek health information, compared with men.22 The over-representation of women is consistent with data from other medicines call centres.23 Nonetheless, the proportion of calls from major cities and urban and remote areas is comparable to the Australian population.24 To our knowledge, no study to date has investigated the level of health literacy of people commonly calling medicines call centres. However, studies have shown that low health literacy is associated with decreased use of preventive services,21 yet higher use of acute healthcare services.25
Data were available between 2002 and 2010, and data on specific consumer concerns – based on the main questions asked – were available between 2007 and 2010. A limitation of this study is that we do not have more recent data. However, concerns raised by consumers in this study were consistent over time and are still relevant today. Callers’ main motivation to call was inadequate information and they desired information to be individualised to their personal circumstances; a mention in a CMI was not enough to address their concerns. This is where GPs or pharmacists can play an important part. Unfortunately, for the majority of callers (83%), we do not know if they desired more information after they raised their concerns with a GP or pharmacist, or if the Medicines Line was contacted as their first source of information.
Implications for general practice
We found that consumers were insecure if symptoms they attribute to statin use require action, and that consumer concerns are not sufficiently addressed by CMI leaflets. GPs are best positioned to target these identified information gaps. The concerns and exemplar questions identified in this study can help develop more consumer-relevant information, which in turn may improve adherence to therapy.
Further steps could include the development of a ‘frequently asked questions and evidence-based answers’ series for GPs and mobile phone/internet applications for consumers to easily access this information using the questions we identified in this study as a starting point.
Table 2. Key concerns and comparison with medication information leaflets (n = 1486)
|
Concerns
|
n
|
%
|
Example questions
|
Answer from AMH
|
Included in CMI?
|
|---|
|
Side effect
|
540
|
36%
|
|
|
|
|---|
|
Musculoskeletal
|
145
|
27%
|
Could simvastatin be causing my muscle pains?
|
The reported incidence of muscle aches is >1%. Severe muscle aches are rare (<0.1%) and dose-related. Risk is increased by illness (ie infection, trauma, metabolic disorder), and certain drug interactions*
|
✔
|
|
GI
|
63
|
12%
|
Does atorvastatin cause GI effects (ie flatulence)?
|
The reported incidence of GI symptoms is >1%
|
✔
|
|
General ADRs
|
46
|
9%
|
What are the side effects of atorvastatin?
|
Common adverse effects include (>1%) myalgia, mild transient GI symptoms, headache, sleep disturbance, dizziness, elevated aminotransferase concentrations. Myopathy and rhabdomyolysis are rare (<0.1%)
|
✔
|
|
Skin (itch and rash)
|
27
|
5%
|
Could simvastatin have caused my rash?
|
Skin itch or rash can occur as a result of angioedema, which is rare (<0.1%)
|
✔
|
|
Fatigue
|
25
|
5%
|
Are any of my medicines causing my tiredness?
|
Fatigue is not reported as an adverse effect, but is possibly related to the common side effect insomnia (>1%)
|
✔
|
|
Memory
|
22
|
4%
|
Would my memory loss be associated with atorvastatin?
|
The reported incidence of memory loss <0.1%
|
No
|
|
Insomnia/nightmares
|
20
|
4%
|
Does rosuvastatin cause insomnia?
|
The reported incidence of sleep disturbance (eg insomnia, nightmares) is >1%
|
✔†
|
|
Liver function
|
20
|
4%
|
Could atorvastatin cause elevated liver function tests?
|
Elevated aminotransferases occur in 0.5–2% of patients. This is dose-dependent and generally responds to a dose reduction
|
✔
|
|
Dizziness
|
17
|
3%
|
Could any of my medicines be causing my dizziness, which leads to falls?
|
The reported incidence of dizziness is >1%
|
✔
|
|
Weight gain
|
11
|
2%
|
Can atorvastatin cause an increase in weight?
|
Weight gain is not reported as an adverse effect
|
No
|
|
Headache
|
9
|
2%
|
Could atorvastatin cause headache?
|
The reported incidence of headache is >1%
|
✔
|
|
Numbness or tingling
|
8
|
1%
|
Can rosuvastatin cause tingling and numbness in her extremities?
|
The reported incidence of paresthesia (eg tingling or numbness) is <0.1%
|
✔
|
|
Other side effects
|
127
|
24%
|
Would any of my partner’s medications be causing hair loss?
|
The reported incidence of alopecia is <0.1%
|
NA
|
|
Interaction
|
414
|
28%
|
|
|
|
|---|
|
Other medicines
|
201
|
49%
|
Can I take colchicine with atorvastatin and candesartan?
|
No interactions were reported for atorvastatin and colchicine or atorvastatin and candesartan
|
✔
|
|
Complementary medicines
|
147
|
39%
|
Can he take echinacea with atorvastatin?
|
No interactions were reported for atorvastatin and echinacea‡
|
✔
|
|
Grapefruit
|
41
|
6%
|
Can I take half a glass of grapefruit juice with simvastatin?
|
Grapefruit juice should be avoided, it may increase the amount of simvastatin in your bloodstream and could increase the risks of adverse effects
|
✔
|
|
Alcohol
|
11
|
3%
|
Can I drink alcohol while on atorvastatin?
|
There are no reported interactions between alcohol and atorvastatin. Statins do not appear to worsen liver disease, but liver disease increases statin concentration, which may increase risk of adverse effects
|
✔
|
|
Other interactions
|
14
|
3%
|
Do certain foods interact with her medicines?
|
Only grapefruit has been reported to interact with statins. It is recommended to avoid grapefruit
|
NA
|
|
General information
|
212
|
14%
|
|
|
|
|---|
|
Indication
|
79
|
49%
|
Do I need to be on simvastatin?
|
The indications for statins are hypercholesterolemia and high risk of coronary heart disease, with or without hypercholesterolemia§
|
✔
|
|
Withdrawal
|
33
|
10%
|
Can I cease any of my medicines, because I am concerned I take too many?
|
Avoid stopping statin if there are symptoms of an ACS because stopping is associated with an increased rate of cardiac events
|
No
|
|
Logistics
|
24
|
6%
|
How can I organise a two-year supply of simvastatin to take to South America?
|
Not discussed – See regulations PBS
|
No
|
|
Contraindications
|
15
|
3%
|
Why should you not take rosuvastatin if you are Asian?
|
People of Asian ancestry should use the lowest starting dose as pharmacokinetic studies indicate that people of Asian ancestry may need lower doses
|
✔
|
|
Constituents
|
8
|
3%
|
Are pravastatin tablets gluten free?
|
Not discussed – See manufacturer package
|
✔
|
|
Other general information
|
53
|
29%
|
What are the risks and benefits with statins treatment?
|
Statins reduce progression of atherosclerosis, improve survival and reduce risk of MI and stroke in patients with cardiovascular disease. Risk for adverse effects is increased by illness, and certain drug interactions
|
NA
|
|
Differences between treatments
|
177
|
12%
|
|
|
|
|---|
|
Generic/cost in general
|
86
|
49%
|
Is there a cheaper option for obtaining my medicines?
|
Not discussed – See PBS website||
|
No
|
|
Which statin is better
|
53
|
30%
|
Can my partner swap between atorvastatin and simvastatin?
|
Atorvastatin and simvastatin are similar and both effective lipid lowering agents. The effectiveness of atorvastatin might be a bit better compared to simvastatin
|
No
|
|
Alternative to statin
|
31
|
18%
|
Is there an alternative to statins for lowering cholesterol?
|
Alternatives include bile acid binding resins and fibrates. Statins are generally first choice in treating hypercholesterolemia, they are the most effective oral LDL lowering agents and they are well tolerated
|
No
|
|
Reimbursement
|
7
|
4%
|
What are the PBS requirements for getting anti-cholesterol medication?
|
Not discussed – See PBS website
|
No
|
|
Dosage
|
115
|
8%
|
|
|
|
|---|
|
Timing of dosage
|
67
|
58%
|
Is it better to take simvastatin at night or in the morning?
|
Shorter-acting statins, such as simvastatin, may be slightly more effective when taken in the evening compared to morning
|
✔
|
|
Correct dose
|
15
|
13%
|
Why would the doctor have increased my wife's atorvastatin dose to 80mg?
|
Because side effects are dose dependent it is advised to start low and increase every four weeks depending on lipid levels and cardiovascular risk
|
✔#
|
|
Missed dose
|
16
|
14%
|
I can't remember if I have taken my simvastatin, should I take one now?
|
Not discussed
|
✔
|
|
Clearance
|
9
|
8%
|
How long before atorvastatin has left body?
|
Not discussed
|
No
|
|
Overdose
|
8
|
7%
|
Will I experience side effects if I accidentally doubled my dose of atorvastatin?
|
Risk for side effects increases with dose
|
±**
|
|
Safety
|
28
|
2%
|
|
|
|
|---|
|
General safety
|
19
|
68%
|
Is atorvastatin safe to take?
|
Statins are generally well tolerated. They should be used with caution in people with hepatic impairment, and renal impairment
|
|
|
Safety of long-term use
|
9
|
32%
|
What are the long-term side effects of atorvastatin?
|
Not discussed
|
No
|
|
ACS, acute coronary syndrome; ADR, adverse drug reactions; AMH, Australian Medicines Handbook; CMI, consumer information leaflet; GI, gastrointestinal; LDL, low-density lipoprotein; MI, myocardial infarction; PBS, Pharmaceutical Benefits Scheme is a program of the Australian Government that provides subsidised prescription medicines. The proportion of key concerns is expressed relative to all statin-related calls for which the question was available electronically (n = 1486); the proportion of specific concerns is expressed relative to the key concern; concerns were only compared to the CMIs of the most commonly prescribed statins (atorvastatin and simvastatin).*Drug interactions with statins that may increase the risk for myopathy or rhabdomyolysis: cyclosporin; fibrates; nicotinic acid; paritaprevir, ritonavir, ombitasvir and dasabuvir. †Drug interactions with simvastatin that may specifically increase the risk for myopathy or rhabdomyolysis: amiodarone; azoles; clarithromycin; diltiazem; Erythromycin; HIV-protease inhibitors; imatinib; simeprevir; tacrolimus; ticagrelor; verapamil. ‡Insomnia was only addressed in the CMI of atorvastatin that was available at the time of our data collection. §Current evidence indicates that the risk of interactions between echinacea supplements and most medications is low (https://nccih.nih.gov). Refer to www.heartfoundation.org.au for guidelines on individual risk. ||See www.pbs.gov.au/browse/brand-premium. #The possible dosages were indicated. **CMIs describe what to do when you take too much, however it did not define the dosage that is considered ‘too much’.
|
Authors
Laura Deckx PhD, MSc, Postdoctoral Research Fellow, Primary Care Clinical Unit, Faculty of Medicine, The University of Queensland, Brisbane, Qld. l.deckx@uq.edu.au
Sanne Kreijkamp-Kaspers MD, PhD, FRACGP, MSc, Senior Lecturer, Primary Care Clinical Unit, Faculty of Medicine, The University of Queensland, Brisbane, Qld
Treasure McGuire PhD, BPharm, BSc, GradDipClinHospPharm, GCHEd, Associate Professor, Faculty of Health & Medical Sciences, Bond University, Gold Coast; Senior Lecturer, School of Pharmacy, The University of Queensland, Brisbane; Assistant Director (Practice and Development), Mater Pharmacy Services, Mater Health Services, Brisbane, Qld
Suzanne Bedford PhD, BSc, Honorary Research Fellow, Mater Research Institute, The University of Queensland, South Brisbane, Qld
Mieke van Driel MD, MSc, PhD, FRACGP, Professor and Head, Primary Care Clinical Unit, Faculty of Medicine, The University of Queensland, Brisbane, Qld
Competing interests: None.
Provenance and peer review: Not commissioned, externally peer reviewed.
Acknowledgements
We would like to acknowledge NPS MedicineWise (formerly National Prescribing Service, Australia), funder of NPS Medicines Line and service provider since July 2010. We would also like to thank Mater Health Services for providing the raw service data. No funding was received for this study.