Hidradenitis suppurativa (HS) is a chronic inflammatory disease that occurs in the intertriginous areas of the body and is characterised by multiple inflammatory nodules, abscess and fistulas appearing in flares and resulting in scarring. Older literature suggests that HS is due to apocrine gland dysfunction; however, it is now recognised to be a follicular occlusive disease. Systemic inflammation is an important component, as are comorbidities that include the metabolic syndrome and androgen dysfunction, and smoking.1 The prevalence of HS is thought to be approximately 1%.2 The clinical course and disease severity are variable.3 HS was traditionally considered to be refractory to medical treatment; however, biologic agents have provided a new treatment paradigm and hope for patients.
HS has profound physical and psychological consequences that affect patients’ quality of life. Currently, the disease is most easily classified using the Hurley staging system (Table 1) .4 Assessment of the individual patient requires a full medical history and physical examination, comorbidity assessment and blood tests for inflammatory markers.1 Referral to specialist dermatology services is often required for moderate and severe cases. We present an evidence-based review and aligning clinical experience in treating patients within a local speciality HS clinic.
Diagnosis
Diagnosis of HS involves identification of the disease and assessment of its comorbidities. Fulfilment of three criteria are necessary for the diagnosis of HS:
- typical lesions (ie deep-seated, painful nodules)
- typical anatomical predilection (ie axillae, groins, perineal and perianal regions, buttocks, infra-mammary and inter-mammary folds)
- chronicity and recurrence of lesions.
Clinical assessment requires full-patient examination to determine the Hurley stage (Table 1).
The majority of patients with HS have comorbidities that require identification, investigation and treatment. The most common comorbidities are listed in Table 2. Smoking is a significant association, and cessation is important for disease control. Approximately one-third of patients with HS have a family history of the disease.5
Epidemiology
Failure to recognise HS by healthcare professionals has resulted in misdiagnosis and mismanagement of many patients, who may present to a variety of healthcare providers. These patients often undergo repeat and unnecessary investigations and procedures; for example, the misdiagnosis of recurrent boils is often followed by inappropriate surgery and antibiotic therapy. This results in substantial financial burden for the patient and healthcare system. The current estimate of HS incidence in the general Australian population is 0.67%, with women more frequently affected than men (ratio 3:1).6,7
Table 1. Hurley grading36
|
Hurley stage
|
Characteristic
|
|---|
|
1
|
Abscess formation: single or multiple
No sinus tracts or scarring
|
|
2
|
Recurrent single or multiple abscesses with sinus tracts and scarring
Single or multiple, widely separated lesions
|
|
3
|
Diffuse or almost diffuse involvement or multiple interconnected tracts and abscesses
|
Table 2. HS-associated comorbidities
|
Comorbidity
|
Percentage of HS patients affected (Liverpool Dermatology Clinic)
|
|---|
|
Obesity
|
61%
|
|
Acne
|
52%
|
|
Hyperlipidaemia
|
45%
|
|
Depression
|
42%
|
|
Insulin resistance
|
39%
|
|
Pilonodal sinus
|
27%
|
|
Polycystic ovary syndrome
|
16%
|
|
Diabetes
|
16%
|
|
Hypertension
|
14%
|
|
Keratosis pilaris
|
12%
|
Pathogenesis and aetiology
HS is a complex disorder and its pathogenesis is unclear.1 Histopathology has shown that the primary event in HS is follicular occlusion.8 Disruption of the hair follicle produces a significant recruitment of inflammatory mediators. Factors contributing to inflammation include the patient’s genotype and smoking status, obesity, adipokine dysregulation and insulin/glucose dysregulation. The importance of the microbiome in patients with HS is increasingly recognised.9,10
Clinical findings, evolution and prognosis
The acute phase of HS consists of painful nodules (eg elevated, solid, palpable lesions) that do not point and are frequently multiple (Figure 1b). Comedones are present throughout all phases of the disease (Figure 1a). Subcutaneous inflammation of contiguous nodules leads to the development of abscesses (ie cavity filled with pus presenting as a hot, red, swollen and painful lump), sinus tracks (ie tunnel or cavity leading to skin surface), fistulae (abnormal channel leading between two cavities or surfaces that drain material such as pus) and scarring (Figures 1c–f). Lymphoedema can occur as a consequence of lymph gland scarring and subsequent obstruction of lymph drainage.11 The natural history of HS is not well documented; however, the disease is progressive in the majority of patients if it remains untreated. Cessation of smoking seems to lead to a reduction in the severity of the disease and some patients go into remission.12,13 In our experience, weight loss may have a similar effect. Given the presence of scarring, there is a real but as yet undefined risk of developing squamous cell carcinoma.14
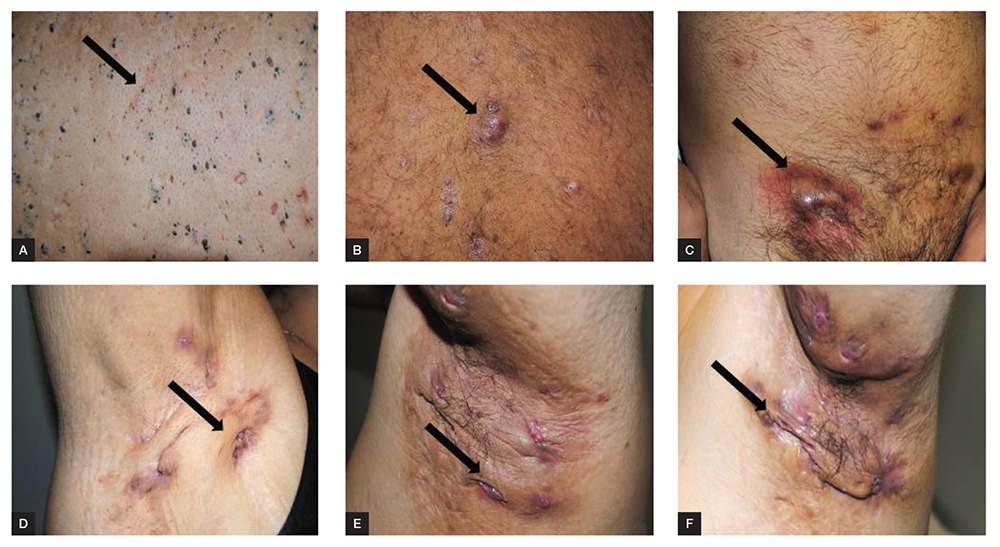
Figure 1. Clinical presentation of hidradenitis suppurativa A, comedone; B, nodule; C, abscess; D, E, sinus; F, fistula
Psychological impact of the disease
The physical appearance, malodorous discharge and painful flare-ups of HS have an understandably negative and distressing emotional impact. Patients are often embarrassed and self-conscious as a result of their inability to control symptoms, and experience stigma from family, friends, partners, the general public and medical community because of a lack of recognition and understanding of the disease. Significant sexual dysfunction is experienced by female and male patients with HS, with females reporting higher sexual distress than males.15 These negative interactions trigger social isolation, anxiety, depression and suicide. Patients with HS have a higher risk of developing depression, and have reported higher depression scores than their matched controls.16 Effective psychological management of HS includes general practitioner (GP) support, referral to specialist psychology and psychiatry services, and enrolment with support groups such as others with HS in Australia.17–19
Pain
Pain is a prominent and debilitating symptom of HS that results in chronic disability, a significant negative psychological impact and impaired quality of life. Pain management is often overlooked and undertreated. No specific approach to pain management in HS is available because of a lack of research and understanding of the underlying mechanisms.20 A variety of agents are reportedly used, including topical analgesics, non-steroidal anti-inflammatory drugs, paracetamol, opioids, gabapentin, pregabalin, duloxetine and venlafaxine. In our clinic, intralesional and oral steroids are used for patients with acute painful lesions who are not responding to other therapies. Depression plays a central role in the perception of pain, and is a risk factor for the development of chronic pain in patients with HS. Pain management in HS is difficult and should be addressed by a multidisciplinary approach; this includes pain assessment, pain scoring, and the involvement of a pain specialist.
Mechanical stress
Mechanical stress has been suggested as a localising factor for HS. Patients with HS report that avoidance of tight‑fitting clothing leads to improvement of symptoms.21
Associations
HS is associated with a number of inflammatory diseases, including those affecting the skin, joints and gut; these include pyoderma gangrenosum, spondyloarthropathies and Crohn’s disease.1 Patients with HS may have one or more signs of the follicular occlusion tetrad (HS, dissecting cellulitis [also called perifolliculitis capitis abscedens et suffodiens], acne conglobata and pilonidal sinus).22
A relationship between HS and Down syndrome has been identified, in which HS has an earlier age of onset, mostly pre‑pubertal, and is often therapy-resistant.23
Approaches to therapy
HS is a complex disorder that requires a holistic approach, including medical management of the disease and its comorbidities (Table 2). Guidelines in the form of an algorithm for treatment have been previously published, and a modified version is illustrated in Figure 2.13,24
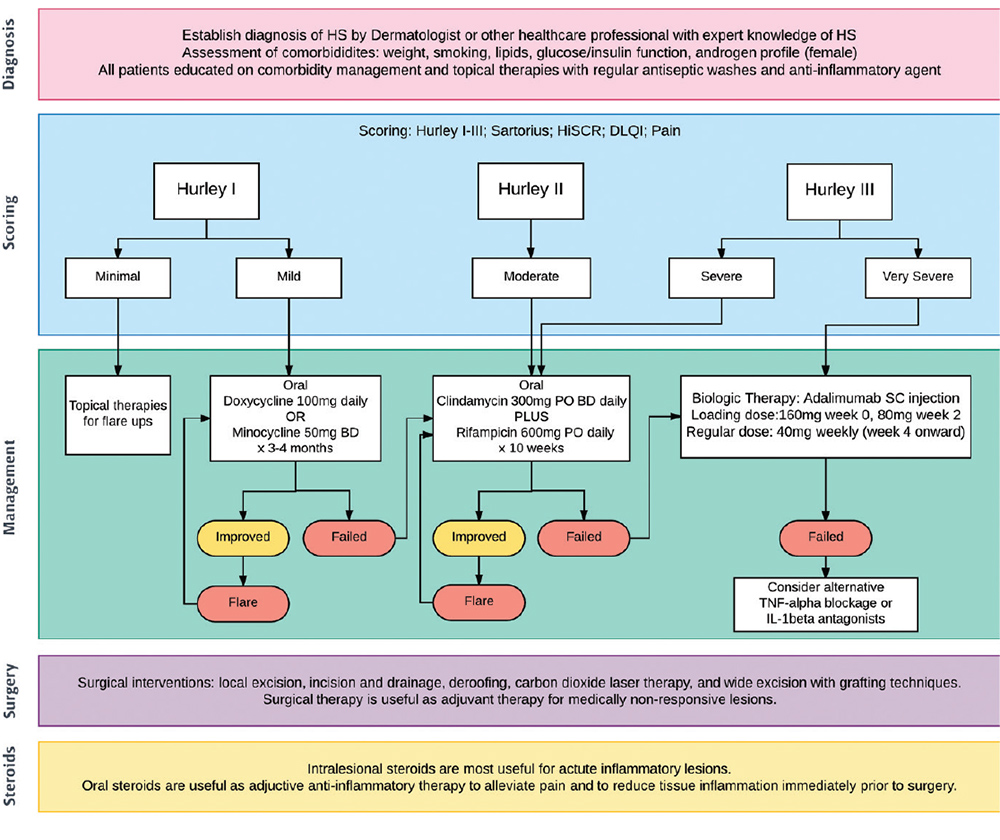
Figure 2. HS treatment algorithm
Patient education about the complex nature of the disease and its comorbidities is essential to ensure treatment compliance. Adjuvant therapy should be offered for pain, weight loss and tobacco cessation at every consultation.24 Monitoring disease staging and blood inflammatory markers is recommended. Referral for specialist consultation is appropriate if first-line therapy fails or HS is severe.
Disease-specific management
Topical management with an antiseptic wash may have a role in HS by decreasing bacterial colonisation.25 In mild HS (Table 1), clindamycin 0.1% solution may be applied to affected areas twice daily for three months; the mechanism of action is probably a bacteriostatic effect.13
Systemic therapy is recommended for moderate-to-severe disease (Table 1) and for those who have not responded to topical treatment. First-line therapy is with systemic antibiotics, while second-line therapies include oral and intralesional corticosteroids, and biologic agents. The efficacy for the use of antibiotics in HS is suggested to be immunomodulatory and anti-inflammatory, not as antibacterial agents.
Recommended antibiotic monotherapy is with minocycline or doxycycline; the efficacy is most probably related to their anti-inflammatory properties.26 Recommended doses are doxycycline 100 mg daily in single or divided doses, or minocycline 100 mg daily. This therapeutic approach is used for a trial period of three months.27
Combination oral antibiotic therapy with clindamycin 300 mg twice daily and rifampicin 600 mg daily for 10 weeks has been shown to be effective for HS in several case series.28 Efficacy is suggested to be immunomodulatory and anti-inflammatory. Adverse events are common and often prevent patients from completing the course.13 The risk of developing colitis due to Clostridium difficile infection is high, especially with longer duration therapy.26
Short-term systemic corticosteroid therapy is highly effective in HS for debilitating pain and acute flares with abscess formation. Their use carries the risk of rebound on withdrawal of therapy among other well-known risks.13 Intralesional corticosteroid with the addition of lignocaine (for management of discomfort) can also be effective in rapidly reducing an acute localised flare.29
Effective biologic agents for HS are largely the tumour necrosis factor alpha inhibitors (anti-TNFs) adalimumab and infliximab.30 Adalimumab is now approved by the Therapeutic Goods Administration for moderate-to-severe HS. Dosing is by weekly subcutaneous injection.31 Infliximab (not approved for this indication) is given by prolonged infusion every six to eight weeks, with dosing based on body weight.13 Anti-TNFs require careful assessment prior to commencing therapy, and monitoring once on treatment in specialised dermatology units. The introduction of these drugs has revolutionised the treatment of HS, and new biologic agents are being developed.
Anti-TNFs are immunosuppressive and health professionals should be aware of the increased risk of infection when using these agents, particularly from Mycobacterium tuberculosis. Live vaccines should not be administered to patients on anti-TNFs at any time.
Surgical intervention
There is no universal agreement about the stage at which surgical intervention should take place. Procedures include local excision, incision and drainage, deroofing, and wide excision with grafting.13 In our experience, surgical intervention is most useful as adjuvant therapy for medically non-responsive lesions, and after a referral for review by a dermatologist experienced in managing HS. Radical wide excision, with extensive removal of affected and unaffected surrounding skin is only necessary in the most extreme cases, and carries significant risk, comorbidity and disfigurement. High recurrence rates have confounded guidelines for surgical intervention in HS.
Laser
Most laser therapy has been directed towards modifying disease activity in the axillae and groin. Theoretically, the destruction of the pilosebaceous apparatus should prevent the extension of HS. Neodymium-doped yttrium aluminium garnet (Nd:YAG) laser and intense pulsed light (IPL) have been used with variable follow-up periods and recurrence rates.13
Comorbidities
Treatment of comorbidities follows standard medical guidelines. Particular attention should be focused towards the investigation of insulin and glucose function, lipid status, and androgen profile in female patients. Patients need to be reviewed regularly to enable early detection and intervention (Table 2). All patients benefit from lifestyle management to ensure their body mass index is within the normal range. Female patients with androgen dysfunction have been shown to benefit from anti-androgen therapy with the oral contraceptive pill, metformin, spironolactone and finasteride; these therapies should be used in accordance with therapeutic guidelines.32–35
Wound care
HS lesions and wounds are often difficult to control because of discharge. Local wound care is useful in preventing unnecessary scarring, psychological embarrassment due to wound suppuration and smell, and pain.13 Use of topical dressings is dependent on lesion type and the extent of disease. All dressings need to limit friction, skin trauma and pain. Absorbent, breathable and non-adhesive dressings are ideal. Optimal dressings should be flexible, allowing conformability, keep wound sites dry and absorb any odour.
Conclusion
HS is a complex medical condition with profound psychological impact. Early disease-modifying intervention is often delayed because of a lack of recognition and understanding among healthcare professionals. Effective treatment is now a realistic goal, but needs to be combined with treatment of the comorbidities and psyche. Thus, GPs play a pivotal role in the holistic treatment of this often devastating disease.
Authors
Dunja A Vekic MBBS, BSc, Ingham Institute for Applied Medical Research, Sydney; Department of Dermatology, Liverpool Hospital, Sydney; University of New South Wales, Sydney. dunja.vekic@sswahs.nsw.gov.au
Geoffrey D Cains PhD, FACD, MBBS, BA (Hons), MA (Hons), Ingham Institute for Applied Medical Research, Sydney; Department of Dermatology, Liverpool Hospital, Sydney; University of New South Wales, Sydney
Competing interests: DV reports grants from Abbvie, grants from Novartis, outside the submitted work. GC reports grants and other from Abbvie, grants from Novartis, grants from Janssen, outside the submitted work.
Provenance and peer review: Not commissioned, externally peer reviewed.
Acknowledgements
The authors would like to acknowledge Associate Professor Pablo Fernández-Peñas, Department of Dermatology, Westmead Hospital, Sydney, New South Wales, Australia; A/Professor Amanda Oakley, Department of Dermatology, Waikato Hospital, New Zealand; Dr Lynda Jane Spelman, Gabba Dermatology and Veracity Clinical Research, Woolloongabba, QLD, Australia.