Type 2 diabetes mellitus (T2DM), previously known as non-insulin dependent diabetes or adult-onset diabetes, is a disorder arising from insulin resistance and relative (rather than absolute) insulin deficiency in the absence of autoimmune beta-cell destruction.1 It is a polygenic disorder involving interactions between genetic and environmental risk factors that result in the underlying pathophysiology of hepatic and muscle insulin resistance, and subsequent beta-cell failure.2 Most patients with this disorder are obese, and T2DM often remains undiagnosed for many years while the patient progresses symptom-free through the earlier stages of hyperglycaemia known as ‘pre-diabetes’. Pre-diabetes includes impaired fasting glucose (IFG; fasting glucose between 5.6 and <7 mmol/L) and impaired glucose tolerance (IGT; two-hour glucose levels between 7.8 and <11.1 mmol/L in the oral glucose tolerance test).1
T2DM in children and adolescents appears to be a more aggressive disease than late-onset T2DM. Progression from IFG or IGT to T2DM in children and adolescents does not necessarily progress linearly with time, and is faster than in adults, generally occurring over 12–21 months.3–5 T2DM and ‘pre-diabetes’ with dysregulated glucose or insulin metabolism is integrally related to obesity, and is increasingly being seen in youth attending specialist obesity services.6
The management of paediatric T2DM is complex as it involves managing the comorbidities associated with diabetes and obesity. Furthermore, treatment options are limited by the lack of licenced treatment modalities in the paediatric population, and adherence, psychosocial health and wellbeing are often poor.7 Early-onset T2DM is associated with significant long-term morbidity and mortality. Adolescents diagnosed with T2DM are predicted to lose 15 years from their remaining life expectancy when compared with their peers who do not have T2DM.8 Complications of diabetes are also common and present even earlier than in adolescents with type 1 diabetes mellitus (T1DM).9,10 A long-term study in Japan found that over a period of 20 years, 24% of the 1063 participants were blind by a mean age of 32 years.11 Another study that followed 426 participants with early-onset T2DM over a mean period of 6.8 years found that 3% required renal dialysis by 35 years of age.12 Acquiring T2DM and its comorbidities at a younger age not only affects an individual’s ability to fully participate in study and work, but also increases morbidity and mortality during the years of peak earning and working capacity.13–15 T2DM in children and youth is therefore a major health issue.
Demographics
The prevalence of T2DM in children and adolescents has increased around the world, in parallel with the increase in the rate of obesity.16–18 In Australia, the incidence of T2DM in patients under the age of 17 years was approximately two per 100,000 person-years, with a 27% rise in average annual adjusted overall incidence between 1990 and 2002.19
Early-onset T2DM most commonly occurs during adolescence and rarely beforehand. Plasma insulin levels rise steadily from pre-pubertal baseline, peaking during puberty then returning to pre-pubertal levels by the third decade of life.20 Insulin sensitivity decreases by around 30% during puberty.21 It is no coincidence therefore that this physiological, puberty-associated insulin resistance coincides with the peak age of early-onset T2DM presentation, and that pre-pubertal T2DM is much less common.9,22 Among the 1.2 million youth in the SEARCH for Diabetes study, there were no children aged 4 years or younger with T2DM, and only 19 cases of children aged 5–9 years with T2DM between 2002 and 2003.23
Diagnosis of T2DM in children and adolescents
The presentation of T2DM varies from asymptomatic hyperglycaemia in a well child, perhaps detected through incidental testing, to ketoacidosis in up to 25% of patients. These individuals are also at risk of hyperglycaemic hyperosmolar non-ketotic state, which is associated with a high mortality rate.24,25 The diagnosis of T2DM in youth requires the diagnosis of diabetes followed by the classification of diabetes type.26 It is worth noting that the prevalence of T1DM is approximately 10-fold higher than the prevalence of T2DM in most paediatric populations. Therefore, a diagnosis of T1DM should be made in the acute setting if there is any doubt regarding diabetes subclassifications.
Delayed treatment of T1DM in youth increases the risk of diabetic ketoacidosis.27,28 Less common causes of diabetes, such as monogenic diabetes due to hepatocyte nuclear factor 4 alpha (HNF4A) and HNF1A mutations (previously known as mature-onset diabetes of the young) and maternally inherited mitochondrial disorders, should be considered in those presenting in adolescence, especially if strong familial clustering of diabetes is present.29
There are currently four accepted ways to diagnose diabetes as per the International Society of Paediatric and Adolescent Diabetes (ISPAD) guidelines and American Diabetes Association, based on the measurement of hyperglycaemia and the presence of symptoms (Box 1).26,30 Several clinical characteristics, such as age, ethnicity, obesity, family history of T2DM and the presence of islet cell antibodies, are important factors in the differentiation between T1DM and T2DM (Table 1).
Box 1. Diagnosis of diabetes defined by the International Society of Paediatric and Adolescent Diabetes (ISPAD) and American Diabetes Association (ADA) guidelines26,30
|
The diagnosis of diabetes is made by the measurement of hyperglycaemia in the absence of any acute physiological stress and the presence of symptoms of hyperglycaemia:
- fasting plasma glucose of ≥7.0 mmol/L
- plasma glucose of ≥11.1 mmol/L post-oral glucose tolerance test, with 1.75 g/kg (max 75g) of anhydrous glucose dissolved in water
- symptoms of diabetes, such as polyuria, polydipsia, nocturia, unexplained weight loss and a random plasma glucose of ≥11.1 mmol/L
- glycated haemaglobin (HbA1c) of >6.5%*
*The test should be performed in a laboratory using a method that certified by the National Glycohemoglobin Standardization Program (NGSP) and standardised to the Diabetes Control and Complications Trial (DCCT) assay. A value of less than 6.5% does not exclude diabetes diagnosed using glucose tests.30,57 The role of HbA1c alone in diagnosing diabetes in children has not been established. |
Table 1. Characteristics of T1DM and T2DM in children and adolescents48
|
| T1DM | T2DM |
|---|
| Age range |
Any age – often young children |
More often seen in peri-pubertal and post-pubertal youth |
| Ethnic distribution |
All groups |
Increased in certain groups (eg Hispanic, Polynesian, Aboriginal and Torres Strait Islander, Indian and Chinese peoples) |
| Sex distribution |
Equal between sexes |
More common in females |
| Symptom duration |
Acute – can be severe |
Often insidious – rarely severe |
| Obesity |
As in population |
Varies according to ethnicity, but up to 85% |
| Family history of diabetes |
Present in 3–5% |
Present in 75–100% |
| Acanthosis nigricans |
12% – can vary in different populations58 |
50–90% – can vary in different populations58 |
| Circulating insulin |
Usually low |
Usually elevated |
| Ketosis at presentation |
More likely to be present |
Less likely, but can be present in up to 25% |
| Islet autoimmunity |
Autoantibodies to insulin (IAA), islet cell cytoplasm (ICA), glutamic acid decarboxylase (GAD), tyrosine phosphatase (insulinoma associated) antibody (IA-2 and IA-2β), or zinc channel antibody (ZnT8) are present at diagnosis in
85–98% of patients with T1DM58,59 |
Usually negative, but can be present in up to 10–20% of patients. If present, islet cell antibodies predict a more rapid requirement of insulin and development of other autoimmune disorders. |
| Associated disorders |
Autoimmune disorders such as autoimmune thyroiditis, coeliac disease |
Other comorbidities of obesity (eg non-alcoholic fatty liver disease, obstructive sleep apnoea, hyperlipidaemia, polycystic ovarian syndrome) |
| Adapted with permission from Sabin MA. Type 2 diabetes in children. Clin Obes 2013;3(3–4):112–6. |
Management of T2DM in children and adolescents
The three main goals in the management of T2DM in children and adolescents are lifestyle modification, normalisation of glycaemia and management of comorbidities (Figure 1).
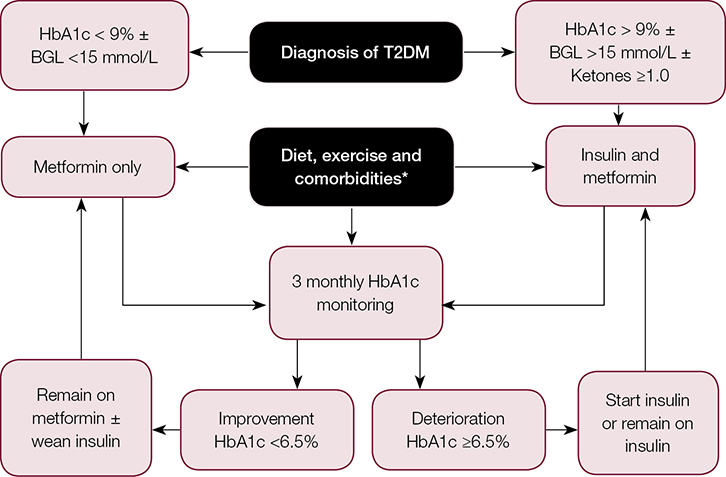 |
|
Figure 1. Management of T2DM in children and adolescents41
*Comorbidities of diabetes and obesity need to be screened at diagnosis. These include:
For diabetes:
- urine microalbuminuria for diabetic nephropathy
- serum lipids for hyperlipidaemia
- blood pressure
- retinal photography for diabetic retinopathy
For obesity:
- liver function tests for non-alcoholic fatty liver disease
- clinical screening for symptoms of obstructive sleep apnoea
- depression
- polycystic ovarian syndrome in adolescent females.
BGL, blood glucose level; HbA1c, glycated haemoglobin; T2DM, type 2 diabetes mellitus
Adapted with permission from Zeitler P, Fu J, Tandon N, Nadeau K, Urakami T, Barrett T, Maahs D: Type 2
diabetes in the child and adolescent. Pediatr Diabetes 2014;15 Suppl 20:26–46.
|
Lifestyle modifications
Although lifestyle (dietary and exercise) modifications are crucial to the management of T2DM, less than 10% of youth with T2DM will reach their glycaemic targets with lifestyle modifications alone.24,31,32 This may be related to high rates of loss to follow-up,33,34 factors relating to socioeconomic status,35 and high associated rates of depression.36 Involvement of an experienced dietitian is necessary.
Dietary recommendations may begin with eliminating sugar-containing soft drinks and juices, increasing fruit and vegetable intake, portion control, and changing family dietary behaviour such as eliminating unhealthy food from the household.37 It is well established that regular exercise without calorie restriction or weight loss is associated with reduced insulin resistance and increased insulin sensitivity in youth who are overweight or obese, regardless of T2DM status.38–40 Exercise recommendations, such as moderate-intensity exercise for 60 minutes daily and reduction in screen time to less than two hours daily, are important parts of the diabetes management plan.41
However, little evidence exists regarding the effectiveness of lifestyle modifications in youth with T2DM. While observational studies suggest higher levels of activity are associated with better glycaemic control in youth with T2DM,42 the only large-scale therapeutic trial to assess lifestyle therapy in 699 youth with T2DM did not support this finding. The study found that 24 months of intensive lifestyle modifications (200–300 minutes of moderate-to-vigorous-intensity exercise per week and 1200–1500 daily calorie intake) on top of metformin monotherapy did not improve long-term glycaemic control at 24 months follow-up.43 Similarly, cardiovascular risk factors, such as dyslipidaemia and inflammatory markers, were not improved by lifestyle modifications on top of metformin monotherapy.44
Normalisation of glycaemia
Youth with confirmed T2DM are usually treated with metformin and/or insulin therapy, which is determined by symptoms, severity of hyperglycaemia and presence of ketosis. Metformin monotherapy is the treatment of choice in those who are metabolically stable (glycated hemoglobin [HbA1c] <9%), beginning with 500 mg daily and titrating to 1000 mg twice daily over four weeks.41 Metformin reduces hepatic gluconeogenesis, increases insulin-stimulated glucose uptake in fat and muscle, and does not usually cause hypoglycaemia.45 An initial anorexic effect may also promote weight loss.45 Long-term metformin use is associated with a 1–2% reduction in HbA1c.41 Insulin therapy is required for those with an HbA1c >9%, severe hyperglycaemia (serum glucose >15 mmol/L), or ketosis/ketoacidosis on presentation (serum ketone ≥1.0 mmol/L and/or serum pH <7.3 ± bicarbonate <15 mmol/L).46
A variety of insulin regimens, such as basal insulin (0.25–0.5 units/kg starting dose) or prandial insulin, are effective in achieving metabolic control.41 Although there are no data to date comparing different insulin regimens in adolescents, this needs to be tailored to the patient’s requirements. Adult studies have shown that a basal glargine regimen is as effective as prandial lispro insulin, with added benefits of simplicity and lower risk of hypoglycaemia.47 It is worth noting that while initiating insulin injections will improve glycaemic control and heighten awareness of diabetes, early insulin therapy may also lead to poorer compliance with treatment, leading to less patient contact and poorer diabetes control in the long term.48 The decision for initiating insulin should therefore be made carefully, balancing the medical benefits with the adolescent and family dynamics.
Other available oral antidiabetic agents are either not approved for use in those aged younger than 18 years or are still under investigation. Sulfonylureas are associated with hypoglycaemia and a greater degree of weight gain (compared with metformin), and may potentially accelerate the loss of beta-cell function.49 Other medications, such as thiazolidinediones, α-glucosidase inhibitors, incretin-mimetics and dipeptidyl peptidase-4 (DPP-IV) inhibitors, although promising, require further investigation as there are no long-term safety data and they are not approved for use in those <18 years of age.41,50
The overall goal of initial treatment is to achieve an HbA1c of <6.5%.41 Education for home blood glucose level (BGL) monitoring is necessary to monitor fasting and postprandial BGLs to achieve target BGL (4–8 mmol/L) and HbA1c ranges (<6.5%). A multidisciplinary approach involving paediatric/adult endocrinologists, diabetes educators, dietitians, social workers and psychologists is required to achieve this, as well as lifestyle changes.
Management of comorbidities
Youth with newly diagnosed T2DM have a high prevalence of complications relating to diabetes and obesity at presentation.10 Reports suggest the presence of hypertension in 10–32% of patients, microalbuminuria in 14–22%, retinopathy in 9.3%, dyslipidaemia in up to 85% and non-alcoholic fatty liver disease in 22% at diagnosis of T2DM in those aged younger than 30 years.34 More aggressive screening for microvascular and macrovascular complications than in youth with T1DM should therefore be performed at diagnosis and regularly thereafter.51 Recommendations are summarised in Table 2.
Table 2. Management guidelines for complications of T2DM in children and adolescents7
|
| Comorbidity | Prevalence | Screening | Management |
|---|
| Hypertension |
Affects up to 36% of youth within 1.3 years of T2DM diagnosis; up to 65% in cross-sectional studies |
At diagnosis and every subsequent clinic visit. Ensure BP is checked using appropriately sized cuff, and plotted against age, gender and height standardised chart60 |
Lifestyle modifications – weight loss, low salt diet, increasing physical activity. Consider ACE inhibitor treatment if lifestyle changes unsuccessful after six months. Angiotensin receptor blocker if ACE inhibitor not tolerated |
| Dyslipidaemia |
Hypertriglyceridaemia is seen in 60–65% of youth with T2DM. Low HDL in 73% of youth with T2DM |
At diagnosis. If normal, monitoring every two years thereafter. Closer follow-up if abnormal |
Dietitian evaluation and dietary modification. If unsuccessful after six months, consider:
- statin therapy if LDL >3.4 mmol/L
- niacin/fibrate therapy if triglycerides >9.3–13 mmol/L and if >10 years of age as risk of acute pancreatitis
|
| Retinopathy |
- 9.3% at diagnosis
- 12.7% have proliferative retinopathy
by 35 years of age
- 23.7% blind by a mean age of
32 years
|
Dilated pupil exam by optometrist or ophthalmologist at diagnosis. Annual examination if normal and more frequent examination if abnormal |
Refer to ophthalmology if evidence of retinopathy. May require laser therapy
if proliferative retinopathy, clinically significant macular oedema or severe non-proliferative retinopathy |
| Nephropathy |
Often present at diagnosis
14–22% in cross-sectional studies |
- Spot urine: 30–299 mg/g
- 24-hour urine: 20–199 mcg/min
- Can be elevated due to smoking, exercise and menstruation
- Exclude orthostatic proteinuria and renal causes
|
- If persistent (>2 samples), start ACE inhibitor therapy even if BP normal
- Aim to normalise microalbuminuria
- Treat hypertension
|
| Depression |
Up to 14.7% and is more common
in girls |
Screen for symptoms of depression at diagnosis and periodically, especially in those with poor glycaemic control and/or frequent visits to emergency department |
Refer to mental health clinician |
| ACE, angiotensin-converting-enzyme inhibitor; BP, blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; T2DM, type 2 diabetes mellitus |
Other comorbidities associated with obesity and insulin resistance, such as polycystic ovarian syndrome in females, obstructive sleep apnoea (OSA), psychiatric illness and orthopaedic problems, are common. The prevalence of OSA can be as high as 60% in youth who are obese,52 with a 12% higher risk of OSA for each 1 kg/m2 rise in body mass index (BMI).53 Similarly, neuropsychiatric comorbidity is common and seen in up to 26% of youth with T2DM, and up to 14.7%, many of whom are girls, have depressive symptoms.54 Adolescents with T2DM reported more depression and had lower quality-of-life scores, compared with adolescents with T1DM.55 Depressed mood is also associated with poorer glycaemic control, higher number of emergency department visits, and poor adherence to diabetic treatment recommendations.7,56 It is crucial, therefore, to screen for these issues at the diagnosis of T2DM, and ongoing yearly surveillance is required.16
Conclusion
T2DM in children and adolescents is a serious medical concern that is more aggressive than the adult-onset form, more challenging to diagnose, and has limited available treatment options. It is also associated with high rates of complications related to diabetes and obesity.
A family-based approach is required to comprehensively address all medical, lifestyle and psychosocial aspects of care, as well as involvement of specific services such as paediatrics, diabetes education, nutrition, psychology and social work as a multidisciplinary team so as to streamline their care to maximise compliance and optimise outcomes.
Authors
Kung-Ting Kao MBChB, FRACP, Clinical Research Fellow, Department of Endocrinology and Diabetes, the Royal Children’s Hospital, Melbourne, VIC
Matthew A Sabin MBBS, FRACP, PhD, Director of Endocrinology, Diabetes and Obesity, the Royal Children’s Hospital, Melbourne, VIC. matt.sabin@rch.org.au
Competing interests: None.
Provenance and peer review: Commissioned, externally peer reviewed.