Hearing loss affects one in six Australians and is projected to rise to one in four by 20501 with our ageing population. The prevalence of hearing loss increases with age and is as high as 33% in Australians aged 50 years or older.2 Hearing impairment in adults is associated with social isolation, depression, occupational disadvantage and cognitive decline.
The initial approach to hearing rehabilitation is the fitting of hearing aids, which have improved significantly over the past two decades. Traditional air conducting hearing aids are appropriate in most patients with bilateral or single-sided sensorineural or conductive hearing loss, and can amplify sound intensity up to 80 Db. Hearing aids are also indicated in patients who do not want surgery, or where surgery is contraindicated because of medical comorbidities. There are, however, patients for whom air conduction aids are either unsuitable or provide inadequate sound amplification. These patients may benefit from the ever-expanding range of implantable auditory enhancing devices (Table 1).
Table 1. Indications for implantable hearing devices7
|
| Bone conducting devices | Cochlear implant |
|---|
Conductive hearing loss
(air–bone gap >30 dB) |
Severe-to-profound sensorineural hearing loss |
Mixed hearing loss
(sensorineural loss up to 65 dB) |
Single-sided deafness |
Congenital malformations
(eg microtia, atresia) |
Severe tinnitus |
| Chronically discharging ears |
|
| Previous radical mastoidectomy |
|
| Single-sided deafness when cochlear implantation is contraindicated |
|
Bone conducting devices
Bone conduction bypasses impaired conduction in the external and middle ear, with sound energy travelling through the bones of the skull directly to the cochlea. This principle, along with osseointegration of a titanium device implanted into the skull, is the basis for the development of bone conducting implants. Bone conducting devices (BCDs) are indicated for patients with mixed or conductive hearing loss and give superior performance to conventional hearing aids when the air–bone gap exceeds 30 dB.3 BCDs are contraindicated in the presence of local infection in the operative site pre-surgery. Patients with single-sided deafness (SSD), defined as total unilateral sensorineural hearing loss, can benefit from bone conduction via contralateral routing of the sound from the deaf ear to the functioning ear. Patients require bone thresholds of 40 dB or better in the ear to be stimulated when the BCD is placed for conductive hearing loss, and 40 dB or better in the contralateral ear if it is being used for SSD. BCDs are categorised as percutaneous or transcutaneous, each having specific advantages and disadvantages. Currently, four devices are available on the Australian therapeutic register.
Percutaneous bone-anchored devices
Percutaneous bone-anchored hearing devices consist of a titanium screw anchored into the mastoid bone attached to the skin-penetrating abutment. Once osseointegration of this unit has occurred, an external sound transducer is attached to the abutment, transmitting the captured sound into vibrations through the device and into the bone. Available devices include the Cochlear Baha Connect System and the Oticon Ponto Bone-anchored Hearing System. Surgical techniques have improved to allow for a single-stage insertion technique, shorter duration of surgical procedure (approximately 30 minutes), and day surgical procedure that can be done under local anaesthesia.4 Audiological outcomes for percutaneous devices are excellent as little sound attenuation occurs because of the direct connection between the sound processor and implant. An additional advantage of percutaneous devices is their safety with all magnetic resonance imaging (MRI) scanners. However, patients do continue to have difficulty with cosmesis and the required daily cleaning of the external abutment. Complications include:4
- minor skin irritation
- soft tissue overgrowth
- skin infection
- abutment dislodgement
- osseointegration failure.
The overall complication rate has markedly decreased from 59% to 23.9%,4 and this rate is represented largely by minor skin reactions.
Transcutaneous bone-anchored devices
Cosmetic concerns and skin reactions around the abutment have led to the development of transcutaneous bone conduction implant systems. These include the external transducer, which is attached via magnetic force to the magnet and the implant placed beneath the skin. The vibrations are transmitted through the soft tissue, which is often thinned in adults at the time of implantation. Available devices include the Cochlear Baha Attract (Figure 1) and Sophono Alpha System. Advantages of these systems include:
- improved cosmetic appearance
- no need for ongoing maintenance of the implant site
- safe with 1.5 Tesla MRI scanners.
 |
Figure 1. Cochlear BAHA Attract, a transcutaneous BCD
Reproduced with permission from Cochlear |
Artefacts from the device does occur and these should be considered if the device is placed for SSD after acoustic neuroma removal, as this requires monitoring with adequate visualisation of the post-surgical area. Complications include skin irritation and pain secondary to pressure from the magnet.5 Audiological outcomes demonstrate that through the transcutaneous system, while still bringing patients to pure tone range of spoken speech (20–40 dB hearing loss), speech perception improvement is less because of sound attenuation in the soft tissue.5 This limits the device to use in patients with 40 dB or better sensorineural hearing component.
The Med-El Bonebridge (Figure 2) is a newer transcutaneous device available, where the transducer is also implanted, allowing for a reduction in the size of the external processor. The processor transmits electromagnetic signal through the intact skin to the transducer, which transfers this to mechanical vibrations through the skull. Theorectically, this design should reduce the dampening effect found in traditional transcutaneous implants, improving audiological outcomes.6 A challenge with this technology is the larger size of the implant, requiring adequate thickness of mastoid bone assessed with a computed tomography (CT) scan pre-operatively. The risk of minor adverse events such as tinnitus, pain and skin infections is 5%.6 Although studies are limited, early assessment has shown the Bonebridge to be audiologically comparable to percutaneous systems and superior to transcutaneous systems.6
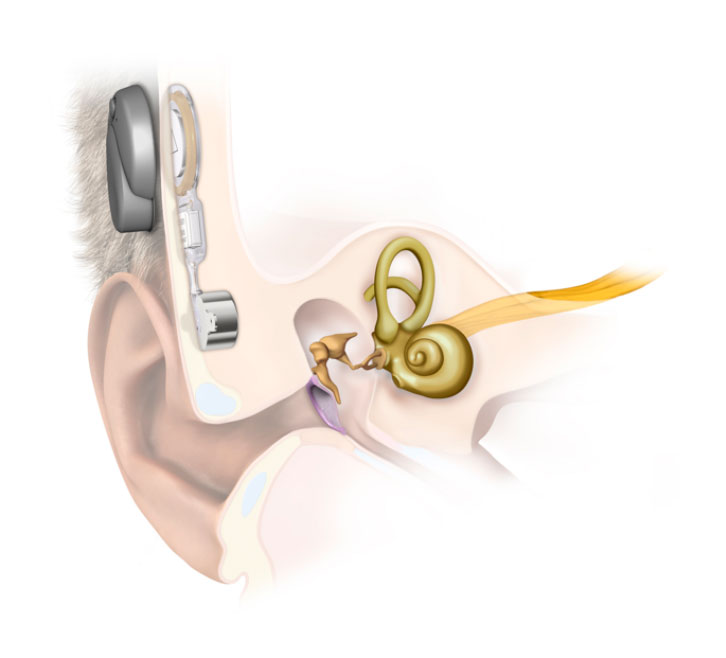 |
Figure 2. Bonebridge, a transcutaneous BCD
Reproduced with permission from Med-El |
Cochlear implants
Cochlear implantation (CI) is a safe and efficacious surgical procedure for hearing rehabilitation in patients with bilateral moderate-to-profound sensorineural hearing loss with inadequate hearing aid amplification. A patient is considered a CI candidate when they score <70% of the correct keywords on open-set, pre-recorded sentence materials presented at 65 dB in the best aided conditions.7 The external components include a sound processor, which is connected to the transmitter. This is magnetically attached to the implanted receiver and stimulator, which converts sound energy into electrical energy and is transmitted to the electrode array within the cochlea. The electrode array replaces the function of hair cells and directly stimulates the cochlea nerve.
Recent developments in CI technology include hybrid devices designed for patients with preserved low frequency hearing (Figure 3). These devices are composed of an acoustic component (hearing aid) for low-frequency hearing stimulation and an electrical component (cochlear implant) for high-frequency stimulation. The external processor can transmit low-frequency sounds to the acoustic component, which amplifies these sounds via the air–bone conduction pathway. Hearing preservation devices use thinner, straight electrodes that cause less trauma to functioning hair cells. Hybrid devices have been successful in preserving residual low frequency hearing.8
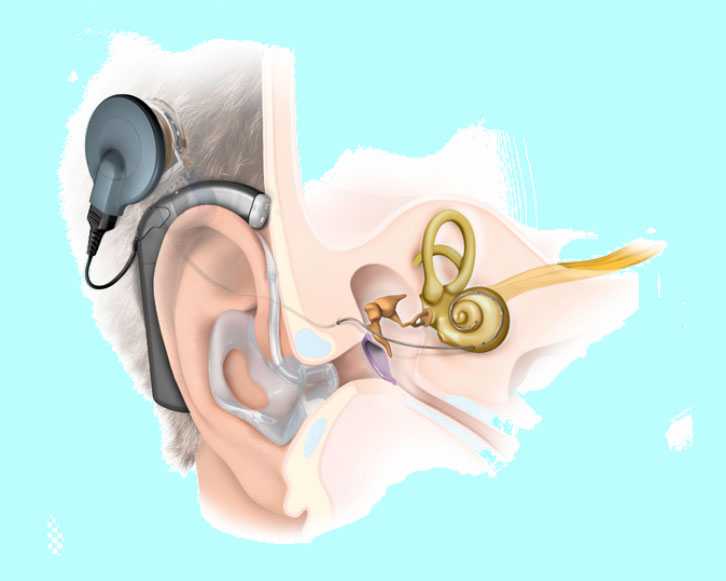 |
Figure 3. Hybrid cochlear implant system
Reproduced with permission from Med-El |
Expanding indications for CI include patients with SSD with an intact cochlear nerve; studies show improvements in speech perception, in noise, sound localisation, tinnitus suppression and in quality of life.9 CI has been shown to reduce tinnitus in patients who experience this in their deaf ear, and some patients report complete resolution when their implant is switched on.9 This has raised the possibility of CI for the treatment of this debilitating condition.
CI is contraindicated in patients with active middle ear disease or absent cochlear nerve. There is no age limit for implantation. Recent studies have demonstrated that advanced age is not an independent risk factor for anaesthetic complications in CI candidates, and complications are not associated with long-term morbidity or mortality.10 It should be highlighted that patients require significant rehabilitation with audiologists and speech pathologists in order to benefit from CI. This should be considered by the cochlear implant team during candidate assessment. The elderly have been shown to gain significant auditory improvement in speech perception and, importantly, gain improvement in psychological, emotional and quality-of-life assessments, leading to increased social participation.10
Overall complication rates have declined steadily over the past two decades from 39% to 9%.11 Improved surgical techniques with smaller incisions and reductions in implant size have largely contributed to reduced adverse events. The most common minor complications include tinnitus and vertigo, which can occur in 10% of patients.11 Major complications are rare, with rates of meningitis reported at 0.4%, and device failure has reduced to <3%.11 In patients requiring MRI scanning, CI is safe with a 1.5 Tesla magnet. With developments in device technology, audiological outcomes continue to improve and CI continues to enhance patient quality of life.12
The role of general practitioners
General practitioners play an integral role in identifying and engaging potential candidates for implantable hearing devices with specialist services. Patients presenting with hearing loss should be assessed for acute aetiologies, such as otitis media, and sudden sensorineural hearing loss requires emergency review and specialist referral for initiation of medical therapy (eg oral and/or intra-tympanic corticosteroids). For those patients with persistent hearing loss who are not coping with conventional hearing aids, consideration of referral to specialist audiological services should be sought.
Authors
Grace Kirkby-Strachan MBBS, Ear Nose and Throat Principal House Officer, Mater Adult Hospital, Brisbane, QLD. grace_ks83@hotmail.com
Christopher Que-Hee MBBS, FRACS, FACS, Otologist/Director Department of Otolaryngology, Head and Neck Surgery, Mater Adult Hospital, Brisbane, QLD
Competing interests: None.
Provenance and peer review: Not commissioned, externally peer reviewed.
Acknowledgements
Cochlear Ltd and Mel-el provided Figures 1–3.