Requesting diagnostic imaging is a common and sometimes challenging task in general practice. About 10% of Australian general practice consultations include new referrals for diagnostic imaging and this proportion is rising.1 International studies of computed tomography (CT) requests from primary care describe inappropriate requests at rates of 12–27%.2,3 A recent report on imaging referrals from Australian GPs found excessive use of referrals for back imaging and ankle ultrasonography. The report expressed concern about the rise in the use of abdominal CT, which would be inappropriate in the absence of ‘red flags’.4
In this article, we use a fictional case to illustrate some principles of prudent diagnostic imaging and offer tips to aid good practice.
Case
On a busy morning in your practice, you receive a call from a local radiologist. One of your general practitioner (GP) colleagues, now on leave, had referred a woman, 30 years of age, for an abdominal CT. The clinical notes read: ‘Abdominal pain – rule out cancer’. Keen to minimise the patient’s exposure to ionising radiation, the radiologist asks if ultrasonography could be used instead as the first investigation. After browsing through the clinical notes, you agree.
‘Appropriate’ imaging – What is it and why does it matter?
Health procedures have been defined as appropriate when ‘the expected health benefit … exceeds the expected negative consequences … by a sufficiently wide margin that the procedure is worth doing, exclusive of cost’.5 Imaging referrals may be inappropriate in several ways:
- when no imaging is indicated
- when imaging is indicated but the incorrect modality or protocol is chosen
- when the timing of imaging is incorrect.
Also, there is an uncertain number of patients who do not undergo imaging when they should.
Why does this matter? First, inappropriate imaging uses money from a finite health budget. Medicare-associated diagnostic imaging already costs Australia more than $3.5 billion annually;6 inappropriate imaging probably contributes to this figure. Second, many imaging procedures involve ionising radiation and sometimes contrast medium exposure. The radiologist in this case is likely to have been concerned about future cancer risks to the patient and irradiation of her ovaries. While such risks are small on an individual level, they add up. In the United States, CT scans performed in a single year were projected to cause 29,000 future cancers.7 Third, false positive and negative results can occur for any investigation, leading to false alarm or inappropriate reassurance. Fourth, imaging may uncover an ‘incidentaloma’.8 A cascade of anxiety, investigations and treatment may follow, which may well shift the cost–benefit and risk–benefit ratios of investigation to adverse levels for the patient. While some incidental findings are important, most are likely to be examples of overdiagnoses – that is, they would not have caused harm if left undiscovered.9
The problem is large. For example, one study found that 9% of abdominopelvic CT scans generated subsequent clinical action after detecting incidental lesions.10 Thyroid nodules may be discovered in at least 50% of adults undergoing carotid Doppler ultrasonography.11 There is a paucity of evidence for the benefit in discovering many of these incidental findings and how best to deal with them is a complex subject even for experts.12
Why does inappropriate imaging occur?
There are many reasons why practice may differ from guidelines.13 This variance is not always inappropriate – sometimes, existing evidence is a poor fit for individual patients. Inappropriate referrals can occur for several reasons, including lack of knowledge of appropriate investigation choice14 and of potential effects of ionising radiation, and have been demonstrated in doctors of many levels of experience.15–17
Further, with technological advances, the perceived importance of the fundamental skills of history-taking and clinical examination may be receding.18 Time constraints and overconfidence in the accuracy and safety of imaging may play a part in this. These basic clinical skills remain vital not just for the maintenance of doctor–patient relationships, but also for reasons of diagnostic precision. Diagnostic reasoning depends on the formation of a provisional diagnosis and pre-test probability (assessed largely from the history and physical examination). Both are necessary to determine the need for imaging, correct modality and correct protocol for any imaging. For example, all abdominopelvic CT scans are not equal – a renal colic CT protocol is entirely different from a protocol to diagnose mesenteric ischaemia. A pre-test probability (in addition to the knowledge of the test’s accuracy) is required to assess the significance of the result of the imaging examination – the post-test probability.19
Another cause of inappropriate imaging may be intolerance of uncertainty by doctors and patients. This is especially common in the setting of somatisation or medically unexplained illness, which are among the most taxing problems managed in general practice. In these situations, we must try to acknowledge our patient’s suffering while not colluding with (and thereby amplifying) their anxiety about the underlying disease.20 This challenge is compounded by fears of adverse medico-legal outcomes should practitioners miss something organic. But finding the correct balance between investigation and reassurance is vital, as iatrogenic harm from over-investigation may be a more common threat than litigation.9,21
Case continued
While your colleague is still on leave, the patient returns to see you for the ultrasonography result, which shows a small, non-specific liver nodule but is otherwise normal. You talk to her about her symptoms and unveil a history consistent with irritable bowel syndrome. She has had many months of recurrent, crampy abdominal pain relieved by defaecation and associated with loose stools. She is anxious about underlying cancer, although there are no red flag signs or symptoms, and there is no iron deficiency. Although the nodule seems unrelated to her symptoms, it triggers a CT, which does not confidently exclude malignancy. A gastroenterologist opinion aided by a magnetic resonance imaging (MRI) finally concludes that the lesion represents focal nodular hyperplasia, a benign condition. Her anxiety is incompletely allayed.
We can only speculate on what led your colleague to order a CT in this case. Perhaps your colleague was unaware that, in the setting of a typical history and absence of red flags, a diagnosis of irritable bowel syndrome can be made without imaging.22 Perhaps he was unaware of the substantial ionising radiation involved in an abdominopelvic CT, which carries approximately a one in 500 chance of malignancy for this patient in future years (Table 1).23 Perhaps his confidence in the likely clinical diagnosis was overwhelmed by the patient’s anxiety about serious illness. In any case, a succession of testing, overdiagnosis and further anxiety ensued. How could this have been averted?
Table 1. Examples of ionising radiation exposures for common imaging procedures23,25
|
| Investigation |
Approximate effective dose (mSv) |
Equivalent period of natural radiation |
Approximate additional risk of cancer in a woman aged 30 years |
| Chest X-ray |
0.02 |
3 days |
1 in 375,000 |
| Lumbar spine X-ray |
1.30 |
7 months |
1 in 5,765 |
| Head CT |
2.30 |
1 year |
1 in 3,259 |
| Abdomen and pelvis CT |
14.0 |
5.8 years |
1 in 535 |
| Nuclear bone scan |
4.0 |
1.6 years |
1 in 1,874 |
| Myocardial perfusion scan |
6.00 |
2.5 years |
1 in 1,249 |
| Ultrasound or MRI |
0 |
0 |
0 |
Avoiding inappropriate imaging – Tips for practice
There are two important and internationally accepted precepts in diagnostic imaging – justification and optimisation.24 The latter means keeping the dose of ionising radiation ‘as low as reasonably achievable’ (the ALARA principle) while still enabling diagnostic-quality imaging. This is the responsibility of imaging technologists and radiologists.
Justification is the decision as to whether the potential benefits of diagnostic imaging outweigh the inherent risks (ie is it appropriate?). It involves considering many of the issues discussed earlier – not just the risks of the procedure itself, but of false alarm and reassurance, incidental results, overdiagnosis and unnecessary expense, and whether the investigation results have a potential to change management. Justification is the shared responsibility of the referring doctor and radiologist. GPs (or other referring doctors) are best placed to assess:
- potential benefit
- potential for change in management
- comorbidities
- risk to their patient of not having the test.
GPs should be aware of the broad principles of radiation risk; that is, risk is generally accepted to be proportional to dose, with no lower threshold below which risk is absent, and increases steeply in younger patients. There are online educational tools available to help revise these principles (see Table 2).
Table 2. Handy online resources
|
|
Diagnostic imaging pathways:
|
|
Calculating cancer risk for a given investigation:
|
|
Imaging information for health consumers:
|
|
General information on radiation risks:
- ARPANSA, www.arpansa.gov.au
- RaysAware, an ionising radiation learning module app for Apple and Android smartphones
|
However, GPs cannot be expected to know all the details of radiological risk to the patient, such as higher versus lower dose imaging protocols. The appropriate protocol is not always the lower dose protocol, but rather the one that minimises radiation exposure while still adequately answering the clinical question posed. The radiologist is in the best position to know these risks and choose protocols, but is hardly ever in a position to adequately assess the potential benefit to an individual patient. The radiologist only knows what is conveyed on the request form. If that information is deficient, how can the imaging specialist be in a position to assess whether the test is justified, which imaging protocol to employ and whether to attach significance to the findings? We suggest that GPs writing request forms include salient clinical findings, questions to be answered, and imaging study being requested. This needs sufficient detail. In our case, the information ‘Abdominal pain – rule out cancer’, while perhaps indicating the patient’s specific anxiety, would not have been useful in choosing a particular CT protocol.
Implicit in the need for sharing responsibility for justification is the need for communication (adequate clinical information from the referrer and advice regarding the choice of imaging from the radiologist) and the need to improve the referrer’s knowledge of the risks of ionising radiation. We encourage GPs and radiologists to view imaging referral as a process of collaboration and consultation (and sometimes gatekeeping by the radiologist) rather than simply ordering and reporting.
Justification and choice of appropriate imaging may be aided by the use of imaging guidelines written for referrers. These need to be evidence-based and developed by consensus with input from radiologists, GPs and patient representatives. Over many years, collaboratively with another team, we have developed a suite of user-friendly guidelines called ‘Diagnostic imaging pathways’. These are freely accessible at www.imagingpathways.health.wa.gov.au and have more recently been released as a free smartphone application (Table 2). Members of our team are researching a version of the pathways combined with electronic requesting and integrated into GP clinical software.
An example of one of our pathways relevant to our irritable bowel syndrome case is shown in Figure 1 (note that the online version of this figure on the website includes much more information). Table 2 also includes other online resources that are helpful to doctors and patients.
These guidelines are literally a guide and are not mandatory rules. They must be flexibly applied so as to account for the complexities and nuances of individual cases. However, we believe they are handy resources for referring doctors.
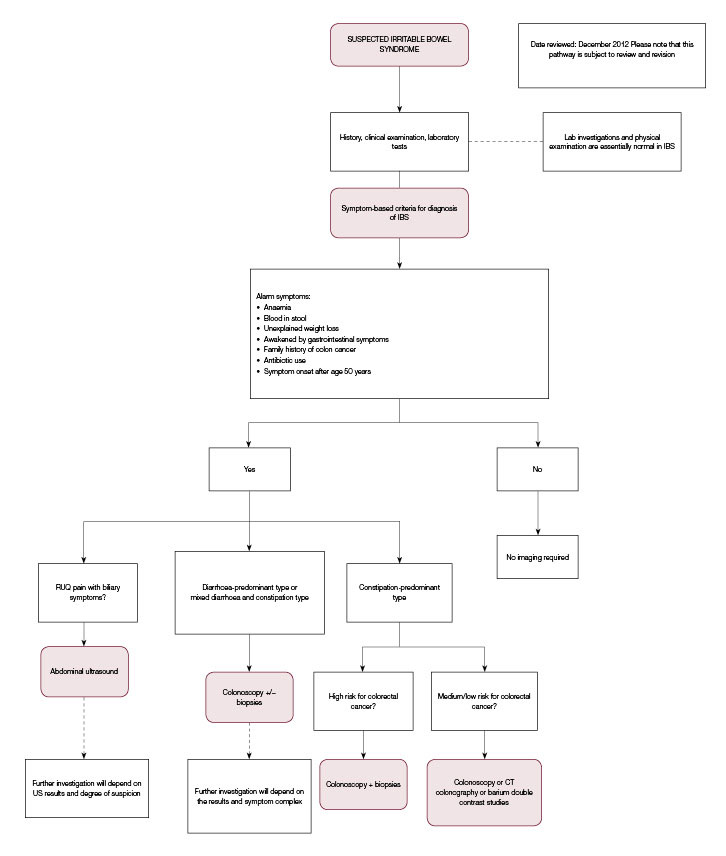 |
Figure 1. ‘Diagnostic imaging pathway’ for suspected irritable bowel syndrome
IBS, irritable bowel syndrome; RUQ; right upper quadrant; US, ultrasound
Reproduced from Diagnostic Imaging Pathways, Department of Health, Western Australia |
Authors
Richard M Mendelson MB, ChB, MRCP, FRCR, FRANZCR, Emeritus Consultant, Royal Perth Hospital; Clinical Professor, School of Surgery, University of Western Australia, Crawley, WA; Adjunct Professor, Notre Dame University, WA
Brett D Montgomery MBBS, DCH, FRACGP, MMedSci, Senior Lecturer, School of Primary Aboriginal and Rural Health Care, University of Western Australia, Crawley, WA. brett.montgomery@uwa.edu.au
Competing interests: RM has been leader of the ‘Diagnostic imaging pathways’ project as part of his paid employment. This is a non-commercial educational and decision support application for referrers to diagnostic imaging. BM is a voluntary member of the ‘Diagnostic imaging pathways’ editorial panel. We promote the ‘Diagnostic imaging pathways’ resource in this article.
Provenance and peer review: Not commissioned, externally peer reviewed.
Acknowledgements
We thank Helen Wilcox and Daniel Hubble for helpful comments on this paper. We acknowledge the support of Western Australia’s Department of Health and the efforts of many individuals in the development of the ‘Diagnostic imaging pathways’ resource.