Significance of NVP
The exact pathophysiology underlying NVP is unknown, but the aetiology is believed to be multifactorial and includes endocrine, gastrointestinal and environmental factors.2 Symptoms of NVP are likely to be more severe in multiple and molar pregnancies. Nausea, with or without vomiting, is experienced by 50–70% of women during early pregnancy.3 This can range from mild, occasional nausea to hyperemesis gravidarum, a condition characterised by intractable vomiting that requires hospitalisation.2 Although mortality secondary to NVP is now rare, there is no doubt that NVP causes significant morbidity. Hospitalisation rates for NVP are second only to premature labour for pregnant women.4 Although overall pregnancy outcome is usually good, severe NVP does increase the risk of low birth weight and preterm birth.5 As NVP after nine weeks’ gestation is rarer, other causes should be considered in any woman presenting with significant symptoms beyond this gestation period.6 In such cases, the differential diagnoses are broad and include co-existent gastrointestinal or genitourinary conditions (eg gastroenteritis, biliary colic or pyelonephritis), neurological conditions (eg migraine) and endocrine disorders (eg hyperthyroidism).
Even for those with mild symptoms, NVP can have a significant impact. In particular, daily function and routine activities are often adversely affected.7–9 One-third to half of all women with NVP require time off work,3,10 and the economical and societal costs include loss of productivity and an increased use of healthcare resources.4 An Australian study in 1999 commented that although the quality of life for women with NVP was similar to that of Australians with chronic illness,10 it often remained untreated or undertreated.
Barriers to NVP treatment
Pharmacotherapy is often seen as a last resort in the treatment of NVP, despite good safety data for the use of antiemetics such as doxylamine, metoclopramide and ondansetron.11–13 Our 2015 study (unpublished data) of 121 Australian women from Sydney’s eastern suburbs found that the majority of women (61%) did not use any NVP treatment. Only 15% had used pharmacotherapy, including those with moderate or severe symptoms on validated testing who had found non-pharmacological options ineffective (Tan A, Lowe S, Henry A, unpublished data).
Historically, clinicians have been wary of prescribing any medications to pregnant women because of concerns about teratogenicity. One study14 indicated that less than 60% of obstetricians and general practitioner (GP) obstetricians prescribed antiemetics to women who requested them.
This reluctance to prescribe may have been reinforced by the thalidomide experience in the 1950s, which resulted in phocomelia in up to 40% of the infants whose mothers had taken it during pregnancy.15 Suspicion and litigation unfortunately extended to other drugs used to treat NVP. A fixed-dose combination of pyridoxine (vitamin B6) and the antihistamine doxylamine has been consistently shown to be very effective in the management of NVP. Several meta-analyses have indicated that there is no teratogenicity associated with its use.16 Despite this, the combination, marketed as Bendectin in the US and Debendox in Australia, was withdrawn from sale in many countries in 1983 following mounting litigation. Tellingly, NVP hospitalisation rates for NVP in the US doubled when pyridoxine/doxylamine use was discontinued.17 However, a generic version (Diclectin) remained on the market in Canada and is still widely used in the treatment of NVP. In 2013, US and Canadian drug regulatory authorities formally re-approved the use of doxylamine succinate 10 mg and pyridoxine hydrochloride 10 mg delayed-release tablets for women whose NVP does not improve with other therapies.
In Australia, no commercial pyridoxine/doxylamine combination is presently available. Doxylamine alone is available as an over-the-counter (OTC) antihistamine in Australia and can be recommended as an off-label treatment for NVP.18,19 Other brands of doxylamine succinate (eg Dozile and Restavit) still carry warning labels against use in pregnancy, despite their categorisation as a Category A medication.20 This can act as a potential barrier to women who might otherwise consider the use of this medication to treat their NVP.21–23
The lack of an imprimatur for the use of doxylamine and the absence of a pyridoxine/doxylamine combination in Australia may also explain the over-reliance on third-line metoclopramide for NVP in this country. One survey of obstetricians and GP obstetricians14 indicated that metoclopramide was the most commonly prescribed treatment for NVP, and was viewed as most effective, despite the paucity of efficacy studies. By contrast, doxylamine was rated as the twelfth most effective treatment14 and is prescribed far less frequently (metoclopramide 70% versus doxylamine 10%).14
Australia lacks NVP-specific management guidelines, which may further complicate local NVP prescribing issues. The National Health and Medical Research Council’s (NHMRC's) Antenatal Care – Module 124 addresses NVP only briefly, noting that ‘although distressing and debilitating for some women, nausea and vomiting usually resolves spontaneously by 16 to 20 weeks pregnancy’. This perhaps reinforces the notion that NVP is unworthy of treatment.
A woman’s own perception of the severity of her symptoms is the most important influence on the management of NVP. This includes whether she chooses to be treated at all, what options she explores, and how long she continues and adheres to treatment.6 It would appear that 24% of women feel that their symptoms are not worth raising with their healthcare practitioner.8 Even when NVP is an acknowledged issue, more than two-thirds of practitioners fail to enquire about the sort of impact it is having on the patient’s quality of life.8 It seems that very few women seek advice on NVP prior to their first scheduled prenatal visit, although 77% of women who are pregnant had experienced NVP before then.8 In fact, a little more than half wait until a subsequent visit to seek advice, by which time the most severe symptoms have probably subsided.8 Early treatment of significant symptoms is important, as it is believed to prevent progression to hyperemesis gravidarum and decrease the likelihood of hospitalisation.1,6
Evidence-based guidelines for NVP management
Undoubtedly, there remains a real need for more high-quality evidence to support one intervention (or suite of interventions) over another. However, this is impeded by difficulties in collating and interpreting the results of studies rather than the interventions being ineffective.25 Despite insufficient strong evidence for any one intervention, as noted by the most recent Cochrane review on NVP,25 this should not preclude effective management of this condition based on the evidence currently available.
Effective management of NVP starts with prevention. Taking prenatal vitamins for three months before conception is recommended as this may reduce the severity of NVP.6 When a woman presents with NVP it is important to explore the physical and psychosocial impact of the symptoms on her life, and reassure her as to the safety of the various treatment options. In those with severe vomiting, or where dehydration or neurological symptoms are present, inpatient investigation and management are warranted.
Treatment is guided by symptom severity, the impact on the woman’s life, maternal–fetal safety profile and patient preference.8 Dietary advice, lifestyle modifications and non-pharmacological remedies, including ginger, tend to be the first choice for most women.17 Such therapies (Figure 1) may help mild NVP, but are less likely to be effective in severe cases.14 As iron tablets may worsen NVP symptoms, suspending the use of supplements/iron-containing multivitamins may be of use.24
When non-pharmacological measures are found not to be effective, progression to pharmacotherapy is warranted.1,6 In the absence of any reliable Australian data or comprehensive local guidelines, those developed by the American College of Obstetricians and Gynecologists (ACOG) in 2015 and Society of Obstetricians and Gynaecologists of Canada (SOGC) in 2002 may be useful and are summarised in Table 1 (available online only). In women with persistent vomiting, the additional use of acid-reducing therapy, such as ranitidine 150–300 mg twice daily orally or rabeprazole 20 mg twice daily orally, should be considered.26
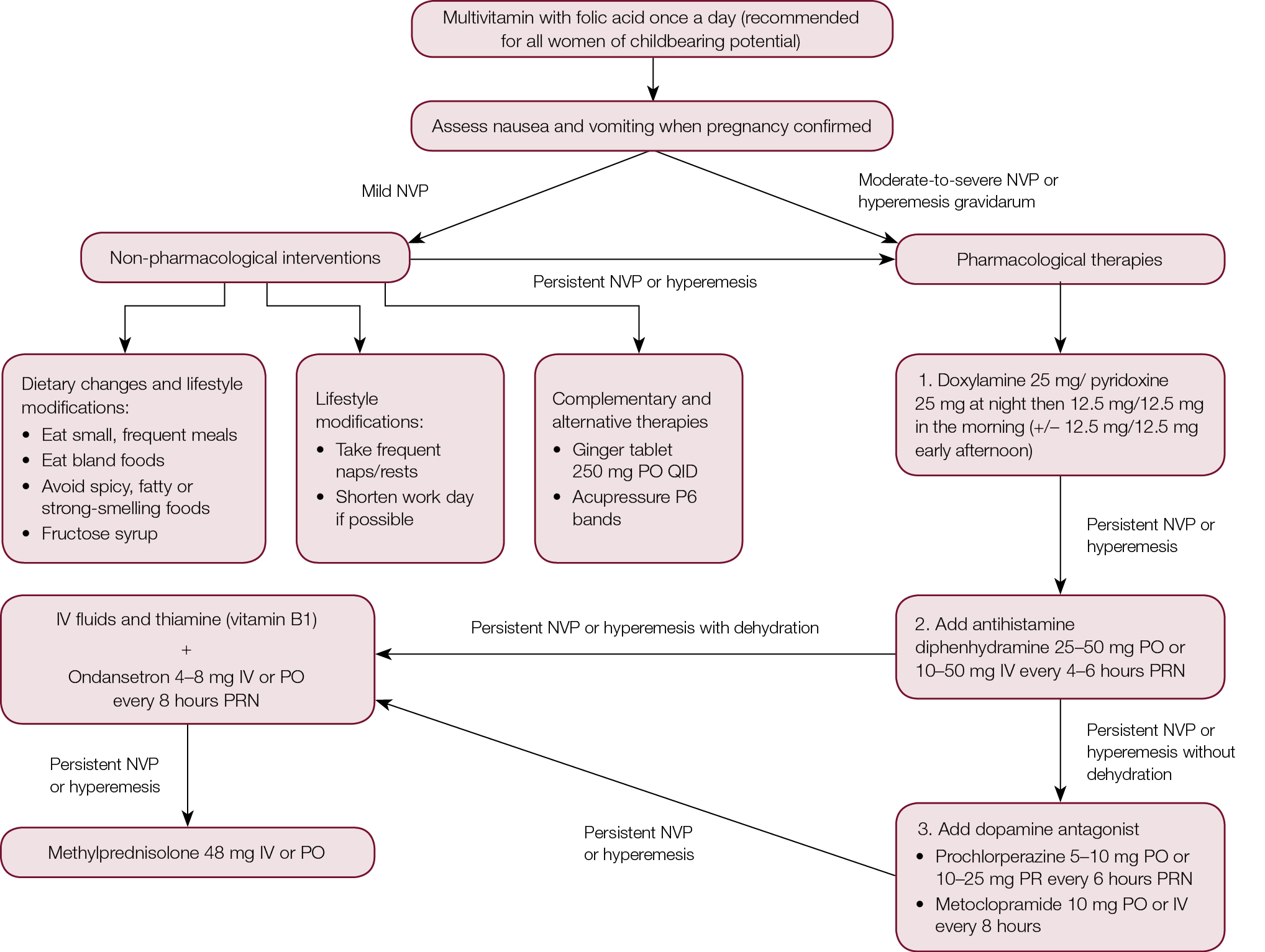 |
| Figure 1. Management algorithm for nausea and vomiting in pregnancy (NVP)5,24,35 |
Summary
The majority of women who are pregnant suffer from NVP and consequent adverse effects on their quality of life. However, this condition is undertreated for many and the impact unacknowledged. A useful algorithm for the management of NVP, incorporating both pharmacological and non-pharmacological interventions, is provided in Figure 1.
Authors
Amelia Tan, medical student, School of Women’s and Children’s Health, University of New South Wales, Sydney, NSW
Therese Foran, MBBS, MClinEd, FAChSHM, Lecturer in Obstetrics and Gynaecology, School of Women’s and Children’s Health, University of New South Wales, Sydney, NSW
Amanda Henry MPH, FRANZCOG, BMed (Hons), BMedSci (Hons), Dip Diagnostic Ultrasound (O&G), Lecturer in Obstetrics and Gynaecology, School of Women’s and Children’s Health, University of New South Wales, Sydney, NSW; Department of Maternal-Fetal Medicine, Royal Hospital for Women, Randwick, NSW; Obstetrician, St George Hospital, Kogarah, NSW. Amanda.Henry@unsw.edu.au
Competing interests: Outside this work, Therese Foran has acted in a consultative capacity, delivered presentations and developed educational activities for various pharmaceutical companies that manufacture products used for contraception or to manage menopausal symptoms or sexually transmissible infections; she has received payment in relation to these activities.
Provenance and peer review: Not commissioned, externally peer reviewed.
Table 1. Pharmacological interventions for nausea and vomiting in pregnancy (NVP)
|
Medications in order of preference according to ACOG/SOGC guidelines5,24
|
Dosage recommendations
|
Practical points/evidence for use
|
Side effects
|
Safety/ADEC drug classification
|
|---|
|
Pyridoxine/
doxylamine combination
|
- Off-licence dosing schedule: 25 mg/25 mg at night, 12.5 mg/12.5 mg in the morning (+/–12.5 mg/12.5 mg early afternoon)
|
- Combination not available in Australia but 25 mg doxylamine and 25 mg pyridoxine tablets available OTC
- Maximum recommended dose 50 mg/day for each
|
- Drowsiness (doxylamine) − may require dosage reduction
- Advise caution with driving/operating machinery until dosage stabilised26
|
|
|
Antihistamines
|
- Diphenhydramine 25–50 mg PO (10–50 mg IV during acute treatment) every 4–6 hours PRN
- Pheniramine 45.3 mg PO TDS
- Promethazine 25 mg PO TDS26
|
|
- Drowsiness − may require dosage reductions if significant
|
- Diphenhydramine: A
- Pheniramine: A
- Promethazine: C – high doses in late pregnancy may cause neonatal EPSE; no reported increase in risk of malformation27,28
|
|
Dopamine antagonists
|
- Prochlorperazine 5–10 mg PO every 6 hours PRN
- Prochlorperazine suppositories 10–25 mg PR every 6 hours PRN
- Metoclopramide 10 mg PO or IV every 8 hours
|
- No RCTs on metoclopramide – limited high-quality data, but some favourable evidence for metoclopramide versus placebo29 and one observational study supporting usefulness in HG30
|
- Drowsiness (common with prochlorperazine and metoclopramide)
- May cause restlessness and insomnia
|
- Metoclopramide: A
- Prochlorperazine: C – high doses in late pregnancy may cause neonatal EPSE
- Phenothiazines: no increased teratogenic risk in large cohort studies and a meta-analysis31,32,33
|
|
Serotonin antagonists
plus IV fluids and thiamine (vitamin B1 – recommended to prevent Wernicke’s encephalopathy )
|
- Ondansetron 4–8 mg IV (acute treatment) or PO every 8 hours PRN
|
- Third-line – limited evidence for use in NVP and potentially significant (but rare) cardiac side effects34
- May be used earlier for more severe symptoms
- Specialist advice recommended before commencement
- Concurrent use of aperients to prevent constipation – tablets preferable to bulking agents as smaller volume is required for swallowing
|
- Maternal cardiac side effects (rare): QT prolongation and Torsade de Pointes
- Constipation and headaches
|
- Ondansetron: B1 – no increased risk of major fetal malformations shown in a large registry study published in 201311
|
|
Corticosteroids
(for refractory NVP)
|
- Methylprednisolone IV or prednisolone PO, 2–3 day initial course
|
- May be useful for refractory NVP but not commonly used or recommended
- Requires supervision of a physician or obstetrician with NVP expertise
|
- Maternal side effects: Possible increased risk of orofacial clefts when used <10 weeks gestation
|
- Methylprednisolone: A – caution advised as animal experiments suggest increased orofacial clefting with first trimester use, but not confirmed in human studies
|
|
ACOG, American College of Obstetricians and Gynaecologists; ADEC, Australian Drug Evaluation Committee, EPSE, extrapyramidal side effects; HG, hyperemesis gravidarum; OTC, over the counter; RCTs, randomised controlled trials; SOGC, Society of Obstetricians and Gynaecologists Canada
|