Type 2 diabetes mellitus (T2DM) is often thought of as a condition that affects adults only. However, the rise in childhood obesity levels has seen T2DM emerge as an issue in children and adolescents. In Australia, the estimated minimum prevalence of T2DM among individuals aged 10–24 years rose from seven per 100,000 in 1993–96 to 18 per 100,000 in 2005–06.1 The true prevalence of T2DM may be significantly underestimated because of under-diagnosis and misclassification, which are likely to be more common in younger age groups where the index of suspicion is often less than in older groups.2
When T2DM develops at a younger age, the increased lifetime exposure to hyperglycaemia is associated with higher rates of microvascular and macrovascular complications, compared with those who develop diabetes later in life.3 Moreover, onset of T2DM at a younger age was found to result in increased rates of developing albuminuria, stroke and ischaemic heart disease, compared with a cohort with type 1 diabetes (T1DM).4 When compared with their counterparts without diabetes, people who develop T2DM between the ages of 15 and 24 years have a lifetime risk of developing microalbuminuria of close to 100%, 20% risk of blindness and are likely to have their life expectancy shortened by 15 years.5 There is also a high psychological burden associated with T2DM in young adults.6
These findings have important implications for lifetime burden of illness and downstream human and economic costs. The rise in T2DM among younger people needs to be understood in the context of the complex interaction between ethnicity, modifiable risk factors for T2DM and social determinants of health. For example, the rising prevalence of T2DM is particularly seen in specific ethnic groups including Aboriginal and Torres Strait Islander, Pacific Islander and Asian populations.7,8 T2DM is often part of the metabolic syndrome (characterised by disturbances of blood glucose, blood pressure and cholesterol levels, as well as body mass index [BMI]), which is also becoming more prevalent among young people.9,10 Socioeconomic status and literacy level are linked to the prevalence of metabolic syndrome and T2DM. Lower socioeconomic status and higher obesity levels11 are associated with a higher risk of T2DM12,13 in Australia and internationally. Literacy levels, which are closely related to income level, have been found to have an impact on glycaemic levels.13
Little is known in Australian general practice about the prevalence of risk factors for T2DM among adolescents.2 General practitioners (GPs) could potentially play an important role in the early detection of risk factors for T2DM among adolescents and in intervening to prevent progression to T2DM. However, screening would need to be acceptable to young people and their parents to avoid any risk of psychological distress or harm, be feasible in practice, and associated with improved outcomes. Ideally, screening would be undertaken in collaboration with schools and other organisations to ensure wide reach into the adolescent population.
The pilot study reported here – Identifying Risk for Diabetes in Adolescence (IRDA) – forms the first part of a planned, wider screening program in a collaboration between GPs and local high schools. The aim of the pilot study was to test the acceptability and feasibility of a brief screening program for T2DM risk factors in adolescents in a general practice in a high-risk, low socioeconomic area of regional Victoria. The brief screening program included a short survey instrument, simple clinical measurements and point-of-care pathology testing.
Methods
One general practice participated in the acceptability and feasibility pilot. This practice was chosen after identifying a community with low socioeconomic status and a high prevalence of diabetes (8%), where the issue of early identification of diabetes would be particularly important. The largest practice in that community was chosen as the pilot site, with a view to extending the project to the only government secondary school in the community if the pilot project suggested this might be valuable.
Active patients aged 13–15 years were contacted by mail and invited to attend a screening visit at the clinic with their parents. Screening times were scheduled during after-school hours to maximise convenience for young people and their parents. Inclusion criteria for the potential participants were:
- age 13–15 years
- active patient at the clinic (ie three visits to the clinic in the past two years)
- able to speak English.
- Exclusion criteria were:
- diagnosed with a terminal illness
- unstable mental state
- previous diagnosis of T1DM or T2DM
- current use of steroids
- insufficient English language proficiency to understand the study materials and give informed consent.
A list of potential participants was extracted from the clinic medical record database by the practice manager and imported into Microsoft Excel spreadsheets, one for females and one for males. A random number generator was used to select 25 potential participants from each gender. The principal clinic GP applied the inclusion and exclusion criteria to the lists. An invitation letter to participate in the pilot study, signed by the GP, was then addressed and sent to the parents or guardians of eligible patients. The process was repeated until a target of 20 screening visits were scheduled. Practice nurses undertook follow-up calls to schedule screening visits for interested participants at the clinic.
Participants attended the clinic with a parent. The parents provided written consent and adolescents provided verbal consent to participate in the study. A practice nurse conducted a brief clinical assessment of risk factors for T2DM (Appendix 1; available online only), including BMI, waist circumference, blood pressure (Omron HEM 7211 BP monitor), point-of-care testing for glycated haemoglobin (HbA1c) and total cholesterol (Roche Cobas b101 Point of Care System).
Participants and one parent each completed a questionnaire (Appendices 2, 3; available online only) addressing diet, exercise, and ethnic and family background suggestive of high T2DM risk. The adolescents’ questionnaire included 11 questions adapted from the NSW schools physical activity and nutrition survey (NSW SPANS).14 This adaptation was undertaken through consensus by the study team. The parents’ questionnaire consisted of eight questions based on the Australian Type 2 Diabetes Risk Assessment Tool (AUSDRISK).15 All of the materials used in the study were developed and targeted at the adolescent population and their parents in socially disadvantaged areas with potentially low literacy levels. As such, all of the materials were tested using the Flesch-Kincaid readability test.16 The materials in this study had a Flesch-Kincaid score of seven or below to ensure readability.
A risk assessment report (Appendix 4; available online only) was generated at the end of the screening visit. Reports consisted of a checklist of normal values against the participant’s recorded value and a tick box for family history of T2DM. In the questionnaires, higher risk was allocated to participants who had a parent or sibling with T2DM, or those from an ethnic background with a high risk of T2DM. Diet and exercise questions were scored on the basis of the potential risk for developing diabetes in the future. This was determined using a pragmatic scoring system based on clinical judgement developed by the investigator team.17 A participant with a total score of 10 or more was considered to be at risk of diabetes. For physical measurements, we used 90th percentile values for the specific age group of the participant as indicative of being at ‘at risk’.18–23 We applied the American Diabetes Association (ADA) criteria to the HbA1c results with:24
- cut-off points of <5.8% (<40 mmol/mol) as normal
- 5.8–6.4% (40–46 mmol/mol) indicating increased risk of T2DM
- >6.5% (48 mmol/mol) suggestive of a diagnosis of T2DM.
A risk assessment report was given to the parent at the conclusion of the screening visit. If any ‘possible risk’ scores were present, a recommendation was made for a GP appointment. Great care was taken to explain that the score indicated only a possible risk of health problems such as T2DM in the future.
Participants and parents were asked to provide feedback about the screening visit at its conclusion (Appendix 5, available online only) and a telephone call was made within a week to elicit further feedback from the parents after they had time to reflect on the screening process and risk assessment report. Notes about the feedback provided were made at the time of the telephone call.
Descriptive statistics were used to summarise the risk scores, HbA1c, cholesterol and anthropometric measures of the participants. We used simple thematic analysis to analyse notes from feedback calls.
Ethics approval for this study was obtained from the Human Research Ethics Committee at the University of Melbourne (Ethics ID 1442068). The pilot study was conducted between May and July 2014.
Results
Our initial mail-out of 50 invitations generated no expressions of interest. On the basis of discussions with the clinic staff, we substantially modified recruitment materials to include a simplified flier clarifying that the screening visit was a free service and would not incur any costs to the family. The next mail-out of 50 invitations generated 22 expressions of interest (44% response rate).
Quantitative data
We screened 22 participants aged 13–15 years (nine males and 13 females). The participants’ characteristics are summarised in Table 1. The maximum number of risk factors for T2DM was seven: BMI, waist circumference, blood pressure, HBA1c, total cholesterol, risk scores for diet and exercise questionnaire, and family background (ethnicity or a family history of diabetes). Nineteen (86%) participants had at least one at-risk value for T2DM, the value being outside the normal range for their gender and age group. Eleven participants (50%) had three or more at-risk values for T2DM (Figure 1).
The most frequently observed risk factors are illustrated in Figure 2. High blood pressure was the most commonly observed risk factor (13 [59%] participants).13 Half (50%) of the participants had a family background (ethnicity or family history) suggesting increased risk of T2DM. Of these participants, seven had a parent currently living with T2DM. Among those seven participants, five had a high BMI and four had a high waist circumference. High BMI and/or waist circumference were commonly observed risk values.
Five participants had HbA1c values above 5.8%, and the highest value observed was 6.4%. Arrangements were made for those participants to be followed up in the general practice by the participant’s GP with endocrinology input as appropriate.
Table 1. Participants’ characteristics and reference values
|
|
Males (n = 9)
|
Females (n = 13)
|
|---|
|
|
Participants*
|
Reference value†
|
Participants
|
Reference value†
|
|
Height (m)
|
159.3 (12.6)
|
–
|
158.4 (8.9)
|
–
|
|
Weight (kg)
|
56.9 (21.1)
|
–
|
58.4 (12)
|
–
|
|
BMI (kg/m2)
|
21.8 (5.2)
|
23.8
|
23.1 (3.7)
|
24.6
|
|
Waist circumference (cm)
|
74.9 (11)
|
79.9
|
76.2 (8.4)
|
77.0
|
|
Systolic blood pressure (mm Hg)
|
116 (13)
|
125
|
121 (15)
|
122
|
|
Diastolic blood pressure (mmHg)
|
72 (14)
|
78
|
65 (11)
|
78
|
|
HbA1c (%)
|
5.6 (0.2)
|
5.8†
|
5.65 (0.3)
|
5.8‡
|
|
HbA1c (mmol/mol)
|
38 (2)
|
40†
|
40 (10)
|
40†
|
|
Total cholesterol (mmol/L)
|
3.79 (0.31)
|
4.9
|
4.51 (0.68)
|
4.9
|
|
Low-density lipoprotein (mmol/L)
|
1.77 (0.55)
|
3.2
|
2.17 (0.67)
|
3.3
|
|
High-density lipoprotein (mmol/L)
|
1.23 (0.18)
|
1.2‡
|
1.52 (0.44)
|
1.2§
|
|
Triglycerides (mmol/L)
|
1.74 (0.95)
|
1.1
|
1.94 (0.87)
|
1.2
|
|
*Mean (SD)
†Reference values are 90th percentile values for an individual aged 14 years unless otherwise indicated; reference values are lower for younger participants. BP values are based on those on the 50th percentile for height.20
‡HbA1c of ≥5.8% or 40mmol/mol equates to an increased risk of type 2 diabetes according to the American Diabetes Association guidelines.
§10th percentile values provided for HDL cholesterol
BMI, body mass index; BP, blood pressure; HBA1c, glycated haemoglobin
|
Qualitative data
We collected brief comments on the acceptability of the screening visit for participants (at the visit) and parents (at the visit and in follow-up call). Feedback was positive overall. Parents and participants generally felt that the visit was an easy and straightforward experience:
Thought it was a great idea and everything was great. – 28482, parent
The questionnaire format seemed acceptable and feasible for most:
It was the quickest questionnaire I’ve done, no troubles at all. – 159537, parent
The finger prick testing was confronting for some of the younger adolescent participant:
Fine until the finger prick. I hated it with every fibre of my being! – 28484, adolescent aged 13
Some comments related to how participants would change their habits following the screening visit. Parents seemed positive about having access to their child’s physical and blood test results:
Good to know [child’s name] needs to work on her diet but is otherwise healthy. – 22267, parent
Learning the blood glucose and cholesterol levels was fantastic – 28484, parent
None of the feedback raised concerns about stigmatisation or psychological distress due to the screening visit.
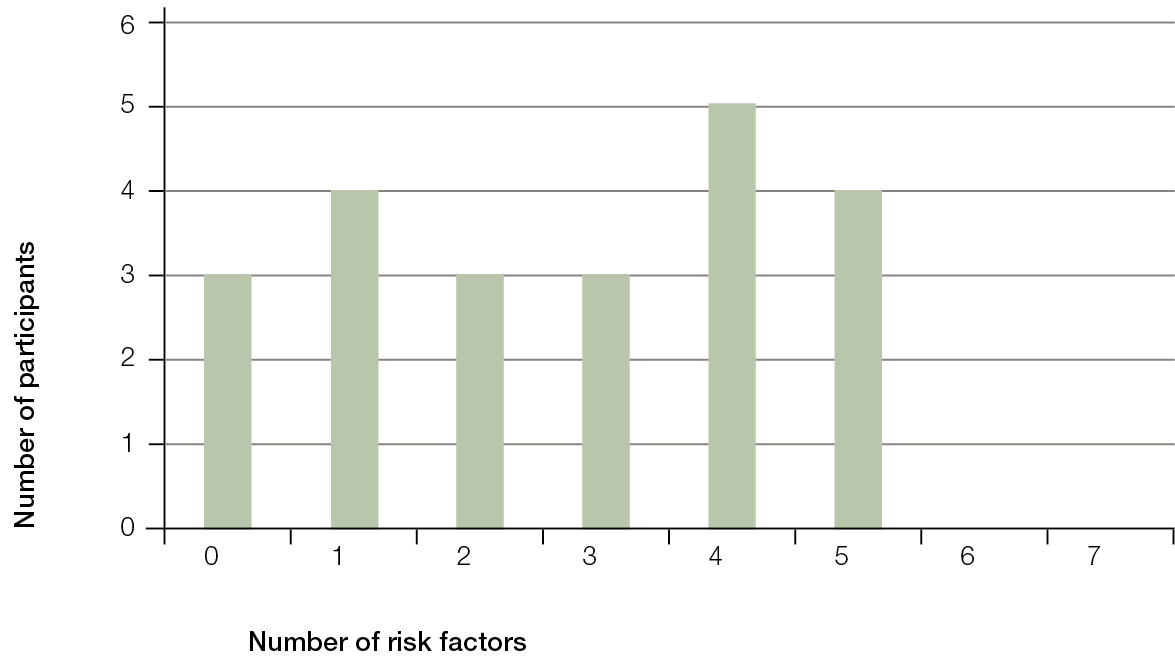 |
| Figure 1. Prevalence of additive risk scores |
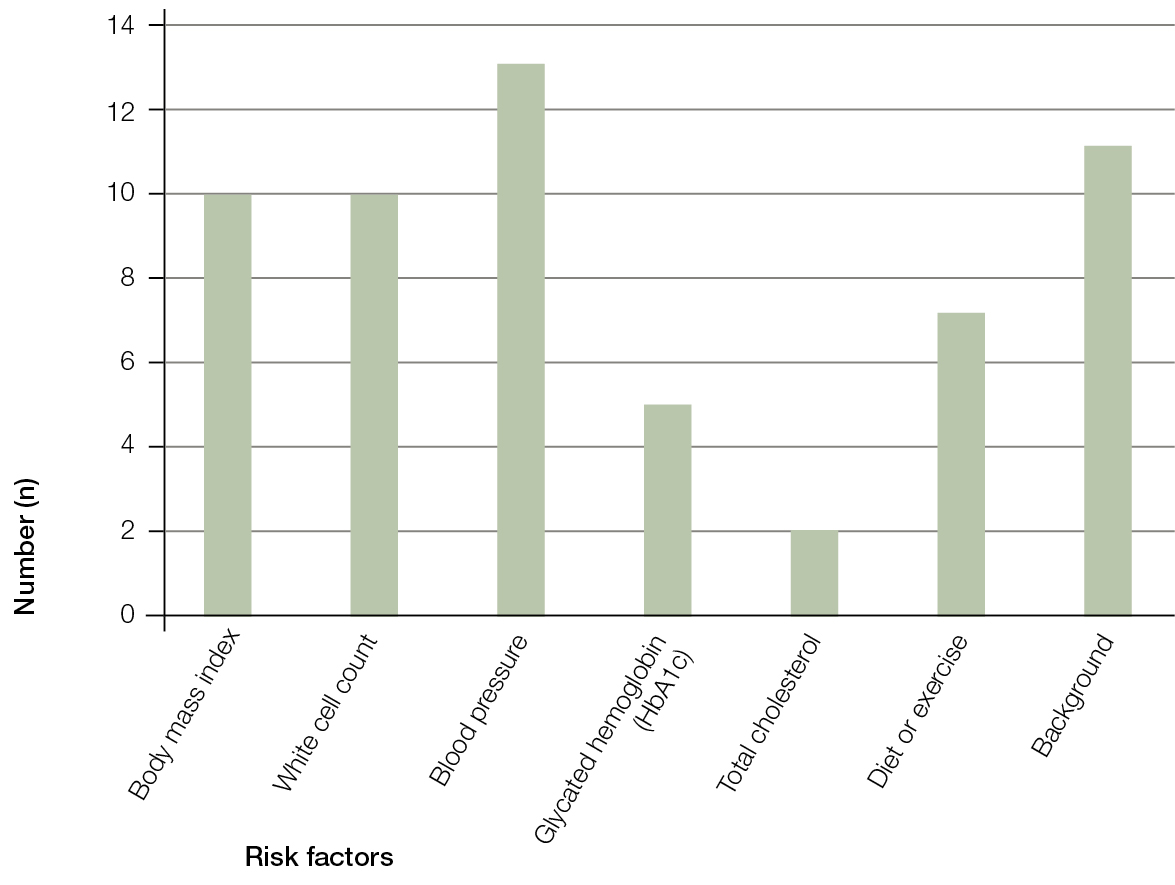 |
| Figure 2. Prevalence of individual risk factors |
Discussion
Our results suggest that screening of adolescents in a general practice setting, in a high-risk population, for T2DM risk factors and dysglycaemia is feasible and acceptable to adolescents and their parents.
Our participants had a high level of behavioural risk factors, with seven cases of at-risk diet and exercise patterns. There were 19 participants who had at least one risk factor and 12 who had three or more risk factors. The prevalence and pattern of these risk factors are similar to trends observed in the NSW SPANS, particularly in low socioeconomic areas. In the NSW SPANS, 26.1% of those who were obese were from low-income areas. The NSW SPANS study did not collect blood glucose measurements or assess the prevalence of pre-diabetes.
Although our small study was not designed to measure the prevalence of risk factors, we did identify a high number of individuals with risk factors of interest. The individuals most at risk for potential T2DM in the future are the five participants with a high HbA1c level, which is indicative of pre-diabetes. This suggests that our open, no-cost-invitation approach in a high-risk area may be useful in targeting adolescents with high individual risk levels. It may be a feasible approach to establishing prevalence data in a supportive setting. In particular, our study did not identify any concerns with stigmatisation or psychological distress as a result of screening. Key factors in our approach may be GP endorsement, providing screening as a free service (as part of a bulk-billing general practice) in a familiar environment and ensuring materials were pitched at an appropriate literacy level.
It is important to acknowledge a number of limitations associated with this pilot study. First, although the adolescents invited to participate in the study were randomly selected, those who chose to participate were self-selected so may not be representative of the practice population. Second, as this was a pragmatic study in a real-world general practice setting, we used point-of-care HbA1c testing to determine diabetes risk. However, it is acknowledged that while guidelines suggest this is an appropriate test, HbA1c may not be as good a predictor of diabetes in adolescents as in adults.25 Finally, we used modified survey items and a scoring system in this study. Further validation, testing of inter-rater and intra-rater reliability and sensitivity analyses will be required before use in a larger prevalence study.
The high number of risk factors observed in this small pilot study suggests a need for extended screening in the future. However, widespread adoption could only be considered after more robust evidence for effectiveness and cost-effectiveness of screening. Our plan is to extend the scope of the screening to a larger population of adolescents in a school-based study in collaboration with local GPs. This could increase reach while maintaining the supportive, familiar primary care environment we used in this pilot study. Our planned school-based study will provide a more reliable estimate of the true prevalence of these risk factors in a low socioeconomic area with a high prevalence of adults with T2DM. It will also provide the basis for establishing longer term outcomes and effectiveness of screening. The rising prevalence of T2DM among adolescents warrants further examination and preventive action.
Authors
John Furler MBBS, DipObst, DCH, GradDip Pub Health, PhD, FRACGP, Principal Research Fellow, Department of General Practice, University of Melbourne, Carlton, Vic. j.furler@unimelb.edu.au
Gareth Rudock BBioMed (Hons), Medical student, Department of General Practice, University of Melbourne, Carlton, Vic
Jo-Anne Manski-Nankervis BSc (Hons), MBBS (Hons), FRACGP, Lecturer, Department of General Practice, University of Melbourne, Carlton, Vic
Irene Blackberry MB, GradCert Ger Nutr, GradCert Health Res and Eval, PhD, Associate Professor, John Richards Initiative, La Trobe University, Wodonga, Vic; and Honorary Associate Professor, Department of General Practice, University of Melbourne, Carlton, Vic
Mark Kennedy BMedSci (Hon), MBBS, GradDip Fam Med, Dip AICD, Clinical Associate Professor, Department of General Practice, University of Melbourne, Carlton, Vic; Corio Medical Clinic, Corio, Vic
Competing interests: Jo-anne Manski-Nankervis has, outside this work, received funding from Sanofi and Roche; payment for lectures from Sanofi; payment for the development of educational presentations from MD Briefcase; and payment from Amgen in relation to a roundtable discussion about a new medication.
Provenance and peer review: Not commissioned, externally peer reviewed